Vicariance, Climate Change, Anatomy and Phylogeny of Restionaceae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chordifex Bgbriggs & Lasjohnson
Flora of Australia Chordifex B.G.Briggs & L.A.S.Johnson Author: Phillip Kodela Cite this profile as: Phillip Kodela (2019) Chordifex. In: Flora of Australia. Australian Biological Resources Study, Department of the Environment and Energy, Canberra. https://profiles.ala.org.au/opus/foa/profile/Chordifex [Date Accessed: 18 March 2019] Generated on Mon Mar 18, 2019 Copyright © Copyright Commonwealth of Australia, 2019 The material in this profile is protected by copyright laws and may be used as permitted under the Copyright Act 1968 or in accordance with licences granted by the copyright owner. Your right to use images and maps or to permit others to use these is subject to the terms of the licence that the contributor of them has applied to the image or map. Information on copyright in images is set out in the Acknowledgements section and through the ALA site at http://www.ala.org.au/faq/using-images-found-on-the-ala/ Text used in this profile has been contributed by the editors and others identified. Unless permitted by the copyright owner, you may download or print a single copy of this material for your own information, research or study. You may not remove any copyright or other notices appearing in this profile. No rights are granted to the Commonwealth Coat of Arms or to any logos or trade marks. Please contact ALA at [email protected] if you believe material in this profile infringes any rights or breaches any contract or licence obligations. License All material CC-BY unless otherwise stated. ISSN: 22077820 Profile Updated: Mon Mar 18, 2019 10:56 AM +11:00 Version: 1 Cite this profile as: Phillip Kodela (2019) Chordifex. -

New Species and Combinations in the African Restionaceae
Available online at www.sciencedirect.com South African Journal of Botany 77 (2011) 415–424 www.elsevier.com/locate/sajb New species and combinations in the African Restionaceae H.P. Linder Institute of Systematic Botany, University of Zurich, Zollikerstrasse 107, CH-8008 Zurich, Switzerland Received 13 January 2010; received in revised form 28 June 2010; accepted 19 October 2010 Abstract Eight new species of the African Restionaceae (Restionoideae) are described, viz.: Cannomois anfracta, Cannomois arenicola, Cannomois grandis, Nevillea vlokii, Thamnochortus kammanassiae, Willdenowia pilleata, Restio uniflorus and Restio mkambatiae. A key to the species of Cannomois is provided, as well as a table comparing the characters of the three species in Nevillea. For all new species, notes on the affinities of the species and their habitats are provided. Two new combinations, Cannomois primosii (Pillans) H.P. Linder and Cannomois robusta (Kunth) H. P. Linder, are made. © 2010 SAAB. Published by Elsevier B.V. All rights reserved. Keywords: Cape Floristic Region; Restionaceae; Restionoideae; South Africa; Taxonomy 1. Introduction variable species can be sensibly divided or from the discovery in the field of species not collected before. Restionaceae are widespread in the Southern Hemisphere, The taxonomy of the African Restionaceae is regularly with a main concentration of species in southern Africa (358 updated and available in the Intkey format, either on a CD avail- species) and Australia (ca. 170 species), and with only one able from the Bolus Herbarium, or as a free download from my species in Southeast Asia and in South America (Briggs, 2001; website at http://www.systbot.uzh.ch/Bestimmungsschluessel/ Linder et al., 1998; Meney and Pate, 1999). -

Jervis Bay Territory Page 1 of 50 21-Jan-11 Species List for NRM Region (Blank), Jervis Bay Territory
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -
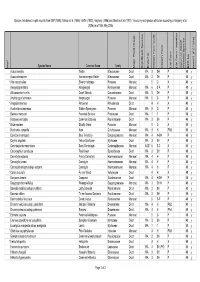
BFS048 Site Species List
Species lists based on plot records from DEP (1996), Gibson et al. (1994), Griffin (1993), Keighery (1996) and Weston et al. (1992). Taxonomy and species attributes according to Keighery et al. (2006) as of 16th May 2005. Species Name Common Name Family Major Plant Group Significant Species Endemic Growth Form Code Growth Form Life Form Life Form - aquatics Common SSCP Wetland Species BFS No kens01 (FCT23a) Wd? Acacia sessilis Wattle Mimosaceae Dicot WA 3 SH P 48 y Acacia stenoptera Narrow-winged Wattle Mimosaceae Dicot WA 3 SH P 48 y * Aira caryophyllea Silvery Hairgrass Poaceae Monocot 5 G A 48 y Alexgeorgea nitens Alexgeorgea Restionaceae Monocot WA 6 S-R P 48 y Allocasuarina humilis Dwarf Sheoak Casuarinaceae Dicot WA 3 SH P 48 y Amphipogon turbinatus Amphipogon Poaceae Monocot WA 5 G P 48 y * Anagallis arvensis Pimpernel Primulaceae Dicot 4 H A 48 y Austrostipa compressa Golden Speargrass Poaceae Monocot WA 5 G P 48 y Banksia menziesii Firewood Banksia Proteaceae Dicot WA 1 T P 48 y Bossiaea eriocarpa Common Bossiaea Papilionaceae Dicot WA 3 SH P 48 y * Briza maxima Blowfly Grass Poaceae Monocot 5 G A 48 y Burchardia congesta Kara Colchicaceae Monocot WA 4 H PAB 48 y Calectasia narragara Blue Tinsel Lily Dasypogonaceae Monocot WA 4 H-SH P 48 y Calytrix angulata Yellow Starflower Myrtaceae Dicot WA 3 SH P 48 y Centrolepis drummondiana Sand Centrolepis Centrolepidaceae Monocot AUST 6 S-C A 48 y Conostephium pendulum Pearlflower Epacridaceae Dicot WA 3 SH P 48 y Conostylis aculeata Prickly Conostylis Haemodoraceae Monocot WA 4 H P 48 y Conostylis juncea Conostylis Haemodoraceae Monocot WA 4 H P 48 y Conostylis setigera subsp. -

The Smut Fungi (Ustilaginomycetes) of Restionaceae S. Lat
MYCOLOGIA BALCANICA 3: 19–46 (2006) 19 Th e smut fungi (Ustilaginomycetes) of Restionaceae s. lat. Kálmán Vánky Herbarium Ustilaginales Vánky (H.U.V.), Gabriel-Biel-Str. 5, D-72076 Tübingen, Germany (e-mail: [email protected]) Received 2 October 2005 / Accepted 25 October 2005 Abstract. Smut fungi of Restionaceae s. lat. were studied. Th ey are classifi ed into two genera, Restiosporium and Websdanea. Problems of species delimitation in these smuts are discussed. In addition to the nine known smut fungi, thirteen new species are described and illustrated: Restiosporium anarthriae, R. apodasmiae, R. chaetanthi, R. desmocladii, R. eurychordae, R. fl exuosum, R. hypolaenae, R. lepyrodiae, R. pallentis, R. patei, R. proliferum, R. spathacei, and R. sphacelatum. Key words: new species, Restionaceae s. lat., Restiosporium, smut fungi, taxonomy, Websdanea Introduction and stained in 1 % aqueous uranyl acetate for 1 h in the dark. After fi ve washes in distilled water, the material was Th e monocotyledonous Restionaceae is a remarkable family dehydrated in acetone series, embedded in Spurr’s plastic, of Southern Hemisphere, evergreen, rush-like plants. Th ey and sectioned with a diamond knife. Semi-thin sections are concentrated mainly in SW Africa and in SW Australia. were stained with new fuchsin and crystal violet, mounted Th e c. 320 species of African Restionaceae, in 19 genera, were in ‘Entellan’ and studied in a light microscope. revised by Linder (1985, 1991). Th e 170 species of Australian Spore ball and spore morphology was studied using Restionaceae s. lat. were published by Meney & Pate (1999) a light microscope (LM) with an oil immersion lens at a in a beautifully illustrated monograph. -

Phylogeny and Subfamilial Classification of the Grasses (Poaceae) Author(S): Grass Phylogeny Working Group, Nigel P
Phylogeny and Subfamilial Classification of the Grasses (Poaceae) Author(s): Grass Phylogeny Working Group, Nigel P. Barker, Lynn G. Clark, Jerrold I. Davis, Melvin R. Duvall, Gerald F. Guala, Catherine Hsiao, Elizabeth A. Kellogg, H. Peter Linder Source: Annals of the Missouri Botanical Garden, Vol. 88, No. 3 (Summer, 2001), pp. 373-457 Published by: Missouri Botanical Garden Press Stable URL: http://www.jstor.org/stable/3298585 Accessed: 06/10/2008 11:05 Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/action/showPublisher?publisherCode=mobot. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit organization founded in 1995 to build trusted digital archives for scholarship. We work with the scholarly community to preserve their work and the materials they rely upon, and to build a common research platform that promotes the discovery and use of these resources. For more information about JSTOR, please contact [email protected]. -

Low Risk Aquarium and Pond Plants
Plant Identification Guide Low-risk aquarium and pond plants Planting these in your pond or aquarium is environmentally-friendly. Glossostigma elatinoides, image © Sonia Frimmel. One of the biggest threats to New Zealand’s waterbodies is the establishment and proliferation of weeds. The majority of New Zealand’s current aquatic weeds started out as aquarium and pond plants. To reduce the occurrence of new weeds becoming established in waterbodies this guide has been prepared to encourage the use of aquarium and pond plants that pose minimal risk to waterbodies. Guide prepared by Dr John Clayton, Paula Reeves, Paul Champion and Tracey Edwards, National Centre of Aquatic Biodiversity and Biosecurity, NIWA with funding from the Department of Conservation. The guides will be updated on a regular basis and will be available on the NIWA website: www.niwa.co.nz/ncabb/tools. Key to plant life-forms Sprawling marginal plants. Grow across the ground and out over water. Pond plants Short turf-like plants. Grow in shallow water on the edges of ponds and foreground of aquariums. Includes very small plants (up to 2-3 cm in height). Most species can grow both submerged (usually more erect) and emergent. Pond and aquarium plants Tall emergent plants. Can grow in water depths up to 2 m deep depending on the species. Usually tall reed-like plants but sometimes with broad leaves. Ideal for deeper ponds. Pond plants Free floating plants. These plants grow on the water surface and are not anchored to banks or bottom substrates. Pond and aquarium plants Floating-leaved plants. Water lily-type plants. -

Special Issue3.7 MB
Volume Eleven Conservation Science 2016 Western Australia Review and synthesis of knowledge of insular ecology, with emphasis on the islands of Western Australia IAN ABBOTT and ALLAN WILLS i TABLE OF CONTENTS Page ABSTRACT 1 INTRODUCTION 2 METHODS 17 Data sources 17 Personal knowledge 17 Assumptions 17 Nomenclatural conventions 17 PRELIMINARY 18 Concepts and definitions 18 Island nomenclature 18 Scope 20 INSULAR FEATURES AND THE ISLAND SYNDROME 20 Physical description 20 Biological description 23 Reduced species richness 23 Occurrence of endemic species or subspecies 23 Occurrence of unique ecosystems 27 Species characteristic of WA islands 27 Hyperabundance 30 Habitat changes 31 Behavioural changes 32 Morphological changes 33 Changes in niches 35 Genetic changes 35 CONCEPTUAL FRAMEWORK 36 Degree of exposure to wave action and salt spray 36 Normal exposure 36 Extreme exposure and tidal surge 40 Substrate 41 Topographic variation 42 Maximum elevation 43 Climate 44 Number and extent of vegetation and other types of habitat present 45 Degree of isolation from the nearest source area 49 History: Time since separation (or formation) 52 Planar area 54 Presence of breeding seals, seabirds, and turtles 59 Presence of Indigenous people 60 Activities of Europeans 63 Sampling completeness and comparability 81 Ecological interactions 83 Coups de foudres 94 LINKAGES BETWEEN THE 15 FACTORS 94 ii THE TRANSITION FROM MAINLAND TO ISLAND: KNOWNS; KNOWN UNKNOWNS; AND UNKNOWN UNKNOWNS 96 SPECIES TURNOVER 99 Landbird species 100 Seabird species 108 Waterbird -

Approved Conservation Advice for Baloskion Longipes
This conservation advice was approved by the Minister / Delegate of the Minister on: 26/3/2008 A statement for the purposes of approved conservation advice (s266B of the Environment Protection and Biodiversity Conservation Act 1999) Approved Conservation Advice for Baloskion longipes This Conservation Advice has been developed based on the best available information at the time this conservation advice was approved. Description Baloskion longipes, Family Restionaceae, previously listed as Restio longipes, is a perennial, grass-like erect sedge with culms (flowering stems) to 150 cm long arising from a compact rhizome (underground stem) (Meney & Pate, 1999; DECC, 2005). This dioecious taxon (male and female flowers on different plants) flowers in summer with seed maturation taking a further 6–8 months (Meney & Pate, 1999). The species is known to resprout after fire (Meney & Pate, 1999). Conservation Status Baloskion longipes is listed as vulnerable. This species is eligible for listing as vulnerable under the Environment Protection and Biodiversity Conservation Act 1999 (Cwlth) (EPBC Act) as, prior to the commencement of the EPBC Act, Restio longipes was listed as vulnerable under Schedule 1 of the Endangered Species Protection Act 1992 (Cwlth). The species is also listed as vulnerable under the Threatened Species Conservation Act 1995 (NSW). Distribution and Habitat Baloskion longipes is known from the Kanangra–Boyd area in the Southern Tablelands (NSW) (DECC, 2005). Populations have been recorded in the Blue Mountains National Park (NP), Kanangra–Boyd NP, Penrose State Forest (in Hanging Rock Swamp), Morton NP (The Vines), the Clyde Mountain area and Ballalaba (south of Braidwood) (DECC, 2005). This species occurs within the Hawkesbury–Nepean, Hunter–Central Rivers, Lachlan and Southern Rivers (NSW) Natural Resource Management Regions (DECC, 2005). -

GENOME EVOLUTION in MONOCOTS a Dissertation
GENOME EVOLUTION IN MONOCOTS A Dissertation Presented to The Faculty of the Graduate School At the University of Missouri In Partial Fulfillment Of the Requirements for the Degree Doctor of Philosophy By Kate L. Hertweck Dr. J. Chris Pires, Dissertation Advisor JULY 2011 The undersigned, appointed by the dean of the Graduate School, have examined the dissertation entitled GENOME EVOLUTION IN MONOCOTS Presented by Kate L. Hertweck A candidate for the degree of Doctor of Philosophy And hereby certify that, in their opinion, it is worthy of acceptance. Dr. J. Chris Pires Dr. Lori Eggert Dr. Candace Galen Dr. Rose‐Marie Muzika ACKNOWLEDGEMENTS I am indebted to many people for their assistance during the course of my graduate education. I would not have derived such a keen understanding of the learning process without the tutelage of Dr. Sandi Abell. Members of the Pires lab provided prolific support in improving lab techniques, computational analysis, greenhouse maintenance, and writing support. Team Monocot, including Dr. Mike Kinney, Dr. Roxi Steele, and Erica Wheeler were particularly helpful, but other lab members working on Brassicaceae (Dr. Zhiyong Xiong, Dr. Maqsood Rehman, Pat Edger, Tatiana Arias, Dustin Mayfield) all provided vital support as well. I am also grateful for the support of a high school student, Cady Anderson, and an undergraduate, Tori Docktor, for their assistance in laboratory procedures. Many people, scientist and otherwise, helped with field collections: Dr. Travis Columbus, Hester Bell, Doug and Judy McGoon, Julie Ketner, Katy Klymus, and William Alexander. Many thanks to Barb Sonderman for taking care of my greenhouse collection of many odd plants brought back from the field. -

Impact of Sea Level Rise on Coastal Natural Values in Tasmania
Impact of sea level rise on coastal natural values in Tasmania JUNE 2016 Department of Primary Industries, Parks, Water and Environment Acknowledgements Thanks to the support we received in particular from Clarissa Murphy who gave six months as a volunteer in the first phase of the sea level rise risk assessment work. We also had considerable technical input from a range of people on various aspects of the work, including Hans and Annie Wapstra, Richard Schahinger, Tim Rudman, John Church, and Anni McCuaig. We acknowledge the hard work over a number of years from the Sea Level Rise Impacts Working Group: Oberon Carter, Louise Gilfedder, Felicity Faulkner, Lynne Sparrow (DPIPWE), Eric Woehler (BirdLife Tasmania) and Chris Sharples (University of Tasmania). This report was compiled by Oberon Carter, Felicity Faulkner, Louise Gilfedder and Peter Voller from the Natural Values Conservation Branch. Citation DPIPWE (2016) Impact of sea level rise on coastal natural values in Tasmania. Natural and Cultural Heritage Division, Department of Primary Industries, Parks, Water and Environment, Hobart. www.dpipwe.tas.gov.au ISBN: 978-1-74380-009-6 Cover View to Mount Cameron West by Oberon Carter. Pied Oystercatcher by Mick Brown. The Pied Oystercatcher is considered to have a very high exposure to sea level rise under both a national assessment and Tasmanian assessment. Its preferred habitat is mudflats, sandbanks and sandy ocean beaches, all vulnerable to inundation and erosion. Round-leaved Pigface (Disphyma australe) in flower in saltmarsh at Lauderdale by Iona Mitchell. Three saltmarsh communities are associated with the coastal zone and are considered at risk from sea level rise. -

Patterns of Flammability Across the Vascular Plant Phylogeny, with Special Emphasis on the Genus Dracophyllum
Lincoln University Digital Thesis Copyright Statement The digital copy of this thesis is protected by the Copyright Act 1994 (New Zealand). This thesis may be consulted by you, provided you comply with the provisions of the Act and the following conditions of use: you will use the copy only for the purposes of research or private study you will recognise the author's right to be identified as the author of the thesis and due acknowledgement will be made to the author where appropriate you will obtain the author's permission before publishing any material from the thesis. Patterns of flammability across the vascular plant phylogeny, with special emphasis on the genus Dracophyllum A thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of philosophy at Lincoln University by Xinglei Cui Lincoln University 2020 Abstract of a thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of philosophy. Abstract Patterns of flammability across the vascular plant phylogeny, with special emphasis on the genus Dracophyllum by Xinglei Cui Fire has been part of the environment for the entire history of terrestrial plants and is a common disturbance agent in many ecosystems across the world. Fire has a significant role in influencing the structure, pattern and function of many ecosystems. Plant flammability, which is the ability of a plant to burn and sustain a flame, is an important driver of fire in terrestrial ecosystems and thus has a fundamental role in ecosystem dynamics and species evolution. However, the factors that have influenced the evolution of flammability remain unclear.