AP3M1 Antibody Catalog # ASC11343
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genome-Wide Analysis of 5-Hmc in the Peripheral Blood of Systemic Lupus Erythematosus Patients Using an Hmedip-Chip
INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 35: 1467-1479, 2015 Genome-wide analysis of 5-hmC in the peripheral blood of systemic lupus erythematosus patients using an hMeDIP-chip WEIGUO SUI1*, QIUPEI TAN1*, MING YANG1, QIANG YAN1, HUA LIN1, MINGLIN OU1, WEN XUE1, JIEJING CHEN1, TONGXIANG ZOU1, HUANYUN JING1, LI GUO1, CUIHUI CAO1, YUFENG SUN1, ZHENZHEN CUI1 and YONG DAI2 1Guangxi Key Laboratory of Metabolic Diseases Research, Central Laboratory of Guilin 181st Hospital, Guilin, Guangxi 541002; 2Clinical Medical Research Center, the Second Clinical Medical College of Jinan University (Shenzhen People's Hospital), Shenzhen, Guangdong 518020, P.R. China Received July 9, 2014; Accepted February 27, 2015 DOI: 10.3892/ijmm.2015.2149 Abstract. Systemic lupus erythematosus (SLE) is a chronic, Introduction potentially fatal systemic autoimmune disease characterized by the production of autoantibodies against a wide range Systemic lupus erythematosus (SLE) is a typical systemic auto- of self-antigens. To investigate the role of the 5-hmC DNA immune disease, involving diffuse connective tissues (1) and modification with regard to the onset of SLE, we compared is characterized by immune inflammation. SLE has a complex the levels 5-hmC between SLE patients and normal controls. pathogenesis (2), involving genetic, immunologic and envi- Whole blood was obtained from patients, and genomic DNA ronmental factors. Thus, it may result in damage to multiple was extracted. Using the hMeDIP-chip analysis and valida- tissues and organs, especially the kidneys (3). SLE arises from tion by quantitative RT-PCR (RT-qPCR), we identified the a combination of heritable and environmental influences. differentially hydroxymethylated regions that are associated Epigenetics, the study of changes in gene expression with SLE. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Supplementary Materials
1 Supplementary Materials: Supplemental Figure 1. Gene expression profiles of kidneys in the Fcgr2b-/- and Fcgr2b-/-. Stinggt/gt mice. (A) A heat map of microarray data show the genes that significantly changed up to 2 fold compared between Fcgr2b-/- and Fcgr2b-/-. Stinggt/gt mice (N=4 mice per group; p<0.05). Data show in log2 (sample/wild-type). 2 Supplemental Figure 2. Sting signaling is essential for immuno-phenotypes of the Fcgr2b-/-lupus mice. (A-C) Flow cytometry analysis of splenocytes isolated from wild-type, Fcgr2b-/- and Fcgr2b-/-. Stinggt/gt mice at the age of 6-7 months (N= 13-14 per group). Data shown in the percentage of (A) CD4+ ICOS+ cells, (B) B220+ I-Ab+ cells and (C) CD138+ cells. Data show as mean ± SEM (*p < 0.05, **p<0.01 and ***p<0.001). 3 Supplemental Figure 3. Phenotypes of Sting activated dendritic cells. (A) Representative of western blot analysis from immunoprecipitation with Sting of Fcgr2b-/- mice (N= 4). The band was shown in STING protein of activated BMDC with DMXAA at 0, 3 and 6 hr. and phosphorylation of STING at Ser357. (B) Mass spectra of phosphorylation of STING at Ser357 of activated BMDC from Fcgr2b-/- mice after stimulated with DMXAA for 3 hour and followed by immunoprecipitation with STING. (C) Sting-activated BMDC were co-cultured with LYN inhibitor PP2 and analyzed by flow cytometry, which showed the mean fluorescence intensity (MFI) of IAb expressing DC (N = 3 mice per group). 4 Supplemental Table 1. Lists of up and down of regulated proteins Accession No. -

Chromosomal Rearrangements Are Commonly Post-Transcriptionally Attenuated in Cancer
bioRxiv preprint doi: https://doi.org/10.1101/093369; this version posted February 1, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Chromosomal rearrangements are commonly post-transcriptionally attenuated in cancer 1 3 1 3, 4, 5 Emanuel Gonçalves , Athanassios Fragoulis , Luz Garcia-Alonso , Thorsten Cramer , 1,2# 1# Julio Saez-Rodriguez , Pedro Beltrao 1 European Molecular Biology Laboratory, European Bioinformatics Institute (EMBL-EBI), Wellcome Genome Campus, Cambridge CB10 1SD, UK 2 RWTH Aachen University, Faculty of Medicine, Joint Research Centre for Computational Biomedicine, Aachen 52057, Germany 3 Molecular Tumor Biology, Department of General, Visceral and Transplantation Surgery, RWTH University Hospital, Pauwelsstraße 30, 52074 Aachen, Germany 4 NUTRIM School of Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, The Netherlands 5 ESCAM – European Surgery Center Aachen Maastricht, Germany and The Netherlands # co-last authors: [email protected]; [email protected] Running title: Chromosomal rearrangement attenuation in cancer Keywords: Cancer; Gene dosage; Proteomics; Copy-number variation; Protein complexes 1 bioRxiv preprint doi: https://doi.org/10.1101/093369; this version posted February 1, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Abstract Chromosomal rearrangements, despite being detrimental, are ubiquitous in cancer and often act as driver events. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Supplementary Table 2
Supplementary Table 2. Differentially Expressed Genes following Sham treatment relative to Untreated Controls Fold Change Accession Name Symbol 3 h 12 h NM_013121 CD28 antigen Cd28 12.82 BG665360 FMS-like tyrosine kinase 1 Flt1 9.63 NM_012701 Adrenergic receptor, beta 1 Adrb1 8.24 0.46 U20796 Nuclear receptor subfamily 1, group D, member 2 Nr1d2 7.22 NM_017116 Calpain 2 Capn2 6.41 BE097282 Guanine nucleotide binding protein, alpha 12 Gna12 6.21 NM_053328 Basic helix-loop-helix domain containing, class B2 Bhlhb2 5.79 NM_053831 Guanylate cyclase 2f Gucy2f 5.71 AW251703 Tumor necrosis factor receptor superfamily, member 12a Tnfrsf12a 5.57 NM_021691 Twist homolog 2 (Drosophila) Twist2 5.42 NM_133550 Fc receptor, IgE, low affinity II, alpha polypeptide Fcer2a 4.93 NM_031120 Signal sequence receptor, gamma Ssr3 4.84 NM_053544 Secreted frizzled-related protein 4 Sfrp4 4.73 NM_053910 Pleckstrin homology, Sec7 and coiled/coil domains 1 Pscd1 4.69 BE113233 Suppressor of cytokine signaling 2 Socs2 4.68 NM_053949 Potassium voltage-gated channel, subfamily H (eag- Kcnh2 4.60 related), member 2 NM_017305 Glutamate cysteine ligase, modifier subunit Gclm 4.59 NM_017309 Protein phospatase 3, regulatory subunit B, alpha Ppp3r1 4.54 isoform,type 1 NM_012765 5-hydroxytryptamine (serotonin) receptor 2C Htr2c 4.46 NM_017218 V-erb-b2 erythroblastic leukemia viral oncogene homolog Erbb3 4.42 3 (avian) AW918369 Zinc finger protein 191 Zfp191 4.38 NM_031034 Guanine nucleotide binding protein, alpha 12 Gna12 4.38 NM_017020 Interleukin 6 receptor Il6r 4.37 AJ002942 -

RNA Splicing in the Transition from B Cells
RNA Splicing in the Transition from B Cells to Antibody-Secreting Cells: The Influences of ELL2, Small Nuclear RNA, and Endoplasmic Reticulum Stress This information is current as of September 24, 2021. Ashley M. Nelson, Nolan T. Carew, Sage M. Smith and Christine Milcarek J Immunol 2018; 201:3073-3083; Prepublished online 8 October 2018; doi: 10.4049/jimmunol.1800557 Downloaded from http://www.jimmunol.org/content/201/10/3073 Supplementary http://www.jimmunol.org/content/suppl/2018/10/05/jimmunol.180055 http://www.jimmunol.org/ Material 7.DCSupplemental References This article cites 44 articles, 16 of which you can access for free at: http://www.jimmunol.org/content/201/10/3073.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision by guest on September 24, 2021 • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2018 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology RNA Splicing in the Transition from B Cells to Antibody-Secreting Cells: The Influences of ELL2, Small Nuclear RNA, and Endoplasmic Reticulum Stress Ashley M. -
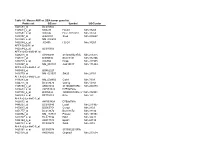
Table S1: Mouse AKR Vs DBA Tumor Gene List Probe Set GB Acc Symbol
Table S1: Mouse AKR vs DBA tumor gene list Probe set GB acc Symbol UGCluster 1425583_at BC010605 1425614_x_at M83244 H2-D1 Mm.33263 1427651_x_at X00246 H2-L /// H2-D1 Mm.33263 1419157_at AI428101 Sox4 Mm.240627 1422565_s_at NM_008688 1452544_x_at J00406 H2-D1 Mm.33263 AFFX-BioB-M_at 1425584_x_at BC010605 AFFX-r2-Ec-bioB-M_at 1426278_at AY090098 2310061N23Rik Mm.271275 1423411_at BI099836 BC013481 Mm.332406 1459725_s_at C86550 Dcpp Mm.287985 1419327_at NM_053181 AA415817 Mm.171484 AFFX-r2-Ec-bioB-3_at 1455869_at BG862223 1416770_at NM_021537 Stk25 Mm.28761 AFFX-r2-Ec-bioB-5_at 1418283_at NM_009903 Cldn4 Mm.7339 1424775_at BC018470 Oas1g Mm.14301 1428850_x_at AK004342 2410026K10Rik Mm.260878 1426633_s_at AW553424 D7Ertd760e 1427932_s_at BI076834 1200003I10Rik /// 1200015M12RikMm.332931 /// 1200016E24Rik 1449289_a_at BF715219 B2m Mm.163 AFFX-r2-Ec-bioC-3_at 1426632_at AW553424 D7Ertd760e 1448207_at BC010840 Lasp1 Mm.271967 1450017_at BG065754 Ccng1 Mm.2103 1451777_at BC013672 BC013672 Mm.33332 1420352_at NM_133731 Prss22 Mm.157351 1423747_a_at BC027196 Pdk1 Mm.34411 1454169_a_at AK017174 Epsti1 Mm.68134 1448793_a_at BC005679 Sdc4 Mm.3815 AFFX-r2-Ec-bioC-5_at 1425161_a_at BC005574 5730502D15Rik 1423158_at AK008566 Gnpnat1 Mm.233534 1453196_a_at BQ033138 Oasl2 Mm.228363 1449250_at NM_033573 Prcc Mm.35089 1452428_a_at AI099111 B2m Mm.163 1421024_at BB524140 Agpat1 Mm.8684 1450016_at BG065754 Ccng1 Mm.2103 1419043_a_at BM239828 AW111922 Mm.326506 1449262_s_at BB704337 Lin7c Mm.235300 1426975_at BG067859 4632413K17Rik Mm.295246 1426164_a_at AF479773 -

C9orf72-Associated SMCR8 Protein Binds in the Ubiquitin Pathway and with Proteins Linked with Neurological Disease John L
Goodier et al. Acta Neuropathologica Communications (2020) 8:110 https://doi.org/10.1186/s40478-020-00982-x RESEARCH Open Access C9orf72-associated SMCR8 protein binds in the ubiquitin pathway and with proteins linked with neurological disease John L. Goodier1*, Alisha O. Soares1, Gavin C. Pereira1, Lauren R. DeVine2, Laura Sanchez3, Robert N. Cole2 and Jose Luis García-Pérez3,4 Abstract A pathogenic GGGCCC hexanucleotide expansion in the first intron/promoter region of the C9orf72 gene is the most common mutation associated with amyotrophic lateral sclerosis (ALS). The C9orf72 gene product forms a complex with SMCR8 (Smith-Magenis Syndrome Chromosome Region, Candidate 8) and WDR41 (WD Repeat domain 41) proteins. Recent studies have indicated roles for the complex in autophagy regulation, vesicle trafficking, and immune response in transgenic mice, however a direct connection with ALS etiology remains unclear. With the aim of increasing understanding of the multi-functional C9orf72-SMCR8-WDR41 complex, we determined by mass spectrometry analysis the proteins that directly associate with SMCR8. SMCR8 protein binds many components of the ubiquitin-proteasome system, and we demonstrate its poly-ubiquitination without obvious degradation. Evidence is also presented for localization of endogenous SMCR8 protein to cytoplasmic stress granules. However, in several cell lines we failed to reproduce previous observations that C9orf72 protein enters these granules. SMCR8 protein associates with many products of genes associated with various Mendelian neurological disorders in addition to ALS, implicating SMCR8-containing complexes in a range of neuropathologies. We reinforce previous observations that SMCR8 and C9orf72 protein levels are positively linked, and now show in vivo that SMCR8 protein levels are greatly reduced in brain tissues of C9orf72 gene expansion carrier individuals. -

Sputum Proteomics and Airway Cell Transcripts of Current and Ex-Smokers with Severe Asthma in U-BIOPRED
Sputum proteomics and airway cell transcripts of current and ex-smokers with severe asthma in U-BIOPRED: an exploratory analysis Kentaro Takahashi 1,2, Stelios Pavlidis3, Francois Ng Kee Kwong 1, Uruj Hoda 1, Christos Rossios1, Kai Sun3, Matthew Loza4, Fred Baribaud4, Pascal Chanez5, Steve J Fowler6, Ildiko Horvath7, Paolo Montuschi8, Florian Singer9, Jacek Musial10, Barbro Dahlen11, Sven-Eric Dahlen11, N. Krug12, Thomas Sandstrom13, Dominic E. Shaw14, Rene Lutter 15, Per Bakke16, Louise J. Fleming1, Peter H. Howarth17, Massimo Caruso18, Ana R Sousa19, Julie Corfield20, Charles Auffray21, Bertrand De Meulder21, Diane Lefaudeux21, Ratko Djukanovic17, Peter J Sterk16, Yike Guo3, Ian M. Adcock1,3, Kian Fan Chung1,3 on behalf of the U-BIOPRED study group# 1National Heart & Lung Institute, Imperial College London, & Biomedical Research Unit, Biomedical Research Unit, Royal Brompton & Harefield NHS Trust, London, United Kingdom; 2Research Centre for Allergy and Clinical Immunology, Asahi General Hospital, Japan; 3Department of Computing & Data Science Institute, Imperial College London, United Kingdom; 4Janssen Research and Development, High Wycombe, Buckinghamshire, United Kingdom; 5 Assistance Publique des Hôpitaux de Marseille - Clinique des bronches, allergies et sommeil, Aix Marseille Université, Marseille, France 6 Centre for Respiratory Medicine and Allergy, Institute of Inflammation and Repair, University of Manchester and University Hospital of South Manchester, Manchester Academic Health Sciences Centre, Manchester, United Kingdom -

Tissue-Infiltrating Macrophages Mediate an Exosome-Based
ARTICLE https://doi.org/10.1038/s41467-019-13894-9 OPEN Tissue-infiltrating macrophages mediate an exosome-based metabolic reprogramming upon DNA damage Evi Goulielmaki1,7, Anna Ioannidou1,2,7, Maria Tsekrekou1,2, Kalliopi Stratigi 1, Ioanna K. Poutakidou1, Katerina Gkirtzimanaki1, Michalis Aivaliotis 1, Konstantinos Evangelou3, Pantelis Topalis1, Janine Altmüller4, Vassilis G. Gorgoulis 3,5,6, Georgia Chatzinikolaou1 & George A. Garinis 1,2* 1234567890():,; DNA damage and metabolic disorders are intimately linked with premature disease onset but the underlying mechanisms remain poorly understood. Here, we show that persistent DNA damage accumulation in tissue-infiltrating macrophages carrying an ERCC1-XPF DNA repair defect (Er1F/−) triggers Golgi dispersal, dilation of endoplasmic reticulum, autophagy and exosome biogenesis leading to the secretion of extracellular vesicles (EVs) in vivo and ex vivo. Macrophage-derived EVs accumulate in Er1F/− animal sera and are secreted in macrophage media after DNA damage. The Er1F/− EV cargo is taken up by recipient cells leading to an increase in insulin-independent glucose transporter levels, enhanced cellular glucose uptake, higher cellular oxygen consumption rate and greater tolerance to glucose challenge in mice. We find that high glucose in EV-targeted cells triggers pro-inflammatory stimuli via mTOR activation. This, in turn, establishes chronic inflammation and tissue pathology in mice with important ramifications for DNA repair-deficient, progeroid syn- dromes and aging. 1 Institute of Molecular Biology and Biotechnology, Foundation for Research and Technology-Hellas, GR70013 Heraklion, Crete, Greece. 2 Department of Biology, University of Crete, Heraklion, Crete, Greece. 3 Department of Histology and Embryology, Athens Medical School, GR11527 Athens, Greece. 4 Cologne Center for Genomics (CCG), Institute for Genetics, University of Cologne, 50931 Cologne, Germany. -

Identification of Novel Regulatory Genes in Acetaminophen
IDENTIFICATION OF NOVEL REGULATORY GENES IN ACETAMINOPHEN INDUCED HEPATOCYTE TOXICITY BY A GENOME-WIDE CRISPR/CAS9 SCREEN A THESIS IN Cell Biology and Biophysics and Bioinformatics Presented to the Faculty of the University of Missouri-Kansas City in partial fulfillment of the requirements for the degree DOCTOR OF PHILOSOPHY By KATHERINE ANNE SHORTT B.S, Indiana University, Bloomington, 2011 M.S, University of Missouri, Kansas City, 2014 Kansas City, Missouri 2018 © 2018 Katherine Shortt All Rights Reserved IDENTIFICATION OF NOVEL REGULATORY GENES IN ACETAMINOPHEN INDUCED HEPATOCYTE TOXICITY BY A GENOME-WIDE CRISPR/CAS9 SCREEN Katherine Anne Shortt, Candidate for the Doctor of Philosophy degree, University of Missouri-Kansas City, 2018 ABSTRACT Acetaminophen (APAP) is a commonly used analgesic responsible for over 56,000 overdose-related emergency room visits annually. A long asymptomatic period and limited treatment options result in a high rate of liver failure, generally resulting in either organ transplant or mortality. The underlying molecular mechanisms of injury are not well understood and effective therapy is limited. Identification of previously unknown genetic risk factors would provide new mechanistic insights and new therapeutic targets for APAP induced hepatocyte toxicity or liver injury. This study used a genome-wide CRISPR/Cas9 screen to evaluate genes that are protective against or cause susceptibility to APAP-induced liver injury. HuH7 human hepatocellular carcinoma cells containing CRISPR/Cas9 gene knockouts were treated with 15mM APAP for 30 minutes to 4 days. A gene expression profile was developed based on the 1) top screening hits, 2) overlap with gene expression data of APAP overdosed human patients, and 3) biological interpretation including assessment of known and suspected iii APAP-associated genes and their therapeutic potential, predicted affected biological pathways, and functionally validated candidate genes.