Professional Fabricare Processes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

First Review - Professional Peers - ITAA Members
DESIGN EXHIBITION COMMITTEE First Review - Professional Peers - ITAA Members Mounted Gallery Co-Chairs: Melinda Adams, University of the Incarnate Word Laura Kane, Framingham State University Su Koung An, Central Michigan University Ashley Rougeaux-Barnes, Texas Tech University Laurie Apple, University of Arkansas Lynn Blake, Lasell College Lynn Boorady, Buffalo State College Design Awards Committee: Melanie Carrico, University of North Carolina, Greensboro Review Chair: Belinda Orzado, University of Delaware Chanjuan Chen, Kent State University Kelly Cobb, University of Delaware Catalog: Sheri L. Dragoo, Texas Woman’s University Sheri Dragoo, Texas Woman’s University V.P. for Scholarship: Youn Kyung Kim, University of Tennessee Rachel Eike, Baylor University Andrea Eklund, Central Washington University Jennifer Harmon, University of Wyoming First Review Erin Irick, University of Wyoming A total of 107 pieces were accepted through the peer review Ashley Kim, SUNY Oneonta process for display in the 2017 ITAA Design Exhibition with Eundeok Kim, Florida State University a 37% acceptance rate. All jurying employed a double blind Helen Koo, Konkuk University process so the jurors had no indication of whose work they Ashley Kubley, University of Cincinnati were judging. A double-blind jury of textile and apparel peers Jung Eun Lee, Virginia Tech reviewed each submission including design statement and YoungJoo Lee, Georgia Southern University images. Further, a panel of Industry experts reviewed submissions Diane Limbaugh, Oklahoma State University -

TRANSPORTATION PROFESSIONALS THIS IS WEAR YOUR BRAND STANDS TALL with Red Kap.®
UNIFORM SOLUTIONS for TRANSPORTATION PROFESSIONALS THIS IS WEAR YOUR BRAND STANDS TALL with Red Kap.® 2 Transportation.RedKap.com BOOST YOUR PROTECT ELEVATE PRODUCTIVITY YOUR TEAM YOUR BRAND KEEP YOUR TEAM FOCUSED ON VISIBILITY SAFETY APPAREL – FOR AN FOR A LOOK THAT STANDS THE JOB. NOT THEIR UNIFORMS. EXTRA LEVEL OF ASSURANCE. THE TEST OF TIME. Pages 4-13 Pages 14-21 Pages 22-31 3 THIS IS WEAR PERFORMANCE MATTERS KEEP YOUR TEAM FOCUSED ON THE JOB. NOT THEIR UNIFORMS. Red Kap® is built to deliver job-inspired performance – giving your team unequalled flexibility and freedom of movement without sacrificing an ounce of comfort. 4 Transportation.RedKap.com PERFORMANCE Red Kap® with Specially designed FLEX PANELS increase your freedom of movement. ELEVATE YOUR BRAND AND BOOST YOUR TEAM’S PERFORMANCE Durable RIPSTOP FABRIC on work ® shirts is 75% STRONGER than UTILIZING RED KAP other poplin work fabrics. WITH MIMIX™ Your business, and your team, are always in motion. Red Kap® with MIMIX™ ensures your PREMIUM, PROFESSIONAL LOOK team is free to focus on the job at hand, not improves customer perception their clothes. Red Kap® with MIMIX™ revolutionizes and fosters employee pride. uniform apparel by providing optimum mobility and comfort for physically active workers, while providing an elevated, professional image for your ™ brand. Our patented MIMIX flex panels greatly MOISTURE WICKING FINISH increase freedom of movement and allow for on work shirts draws moisture breathability where it’s needed most. away from the body. Put it to the test today and discover the Red Kap® with MIMIX™ difference. PERFORMANCE FLEX FABRIC provides ADDITIONAL BREATHABILITY Bend, stretch, squat and reach where you need it most. -

Sew Any Fabric Provides Practical, Clear Information for Novices and Inspiration for More Experienced Sewers Who Are Looking for New Ideas and Techniques
SAFBCOV.qxd 10/23/03 3:34 PM Page 1 S Fabric Basics at Your Fingertips EW A ave you ever wished you could call an expert and ask for a five-minute explanation on the particulars of a fabric you are sewing? Claire Shaeffer provides this key information for 88 of today’s most NY SEW ANY popular fabrics. In this handy, easy-to-follow reference, she guides you through all the basics while providing hints, tips, and suggestions based on her 20-plus years as a college instructor, pattern F designer, and author. ABRIC H In each concise chapter, Claire shares fabric facts, design ideas, workroom secrets, and her sewing checklist, as well as her sewability classification to advise you on the difficulty of sewing each ABRIC fabric. Color photographs offer further ideas. The succeeding sections offer sewing techniques and ForewordForeword byby advice on needles, threads, stabilizers, and interfacings. Claire’s unique fabric/fiber dictionary cross- NancyNancy ZiemanZieman references over 600 additional fabrics. An invaluable reference for anyone who F sews, Sew Any Fabric provides practical, clear information for novices and inspiration for more experienced sewers who are looking for new ideas and techniques. About the Author Shaeffer Claire Shaeffer is a well-known and well- respected designer, teacher, and author of 15 books, including Claire Shaeffer’s Fabric Sewing Guide. She has traveled the world over sharing her sewing secrets with novice, experienced, and professional sewers alike. Claire was recently awarded the prestigious Lifetime Achievement Award by the Professional Association of Custom Clothiers (PACC). Claire and her husband reside in Palm Springs, California. -
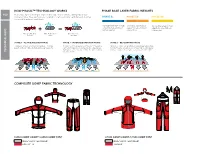
How Phasic™ Technology Works
PMS 301 PMS Process Blue PMS 110 PMS 107 PMS 301 PMS Process Blue HOW PHASIC™ TECHNOLOGY WORKS PHASE BASE LAYER FABRIC WEIGHTS 180 Phasic™ base layer technology is engineered for superior performance during stop-and-go interval activities. Phase garments are designed to retain less moisture and dry faster, keeping PHASE SL PHASE SV PHASE AR the user drier and more comfortable. PMS 301 Superlight base layer for high SeverePMS 152 base layer for high All-roundPMS 110 base layer for high PMS Process Blue output interval activities in outputPMS 136 interval activities in outputPMS 107 interval activities in warmer weather. cold weather. cold weather. Moisture Wicking 100% Hydrophobic Bi-component Phi Yarns Yarns Structure STAGE 1 - ACTIVE/WICKING PHASE STAGE 2 - INTENSE/DISPERSION PHASE STAGE 3 - REST/DRYING PHASE Entering an active phase, Phi yarns rapidly pull moisture Phi yarns work to disperse moisture across the entire Hydrophobic yarns, along with the broadly dispersed moisture away from the skin while the hydrophobic yarns stay dry. garment, helping to regulate body temperature. PMS 110 from the Phi yarns, combine to allow Phasic™ fabric to dry quickly Hydrophobic yarns stay dry and limit the fabric’s ability PMS 107 during a rest phase, keepingPMS theCool user Gray warm 8 and comfortable.PMS 152 TECHNICAL INFO to hold moisture. PMS Cool Gray 3 PMS 136 PMS 152 PMS 136 PMS Cool Gray 8 PMS Cool Gray 3 COMPOSITE GORE® FABRIC TECHNOLOGY PMS Cool Gray 8 PMS Cool Gray 3 ALPHA COMP HOODY / ALPHA COMP PANT LITHIC COMP HOODY / LITHIC COMP PANT GORE® FABRIC TECHNOLOGY GORE® FABRIC TECHNOLOGY FORTIUS™ 1.0 TRUSARO™ MAPP-MERINO ADVANCED INSULATED STORMHOOD™ ROLLTOP™ CLOSURE TECHNOLOGIES AND PERFORMANCE PROGRAM Insulated hood designed for full weather protection. -

CISTM8 President's Letter
Newsletter of the International Society of Travel Medicine 2003 January/February CISTM8 Only 4 months away! President’s Letter he International Society of Travel President’s Letter Medicine will hold its 8th regular January 2003 Tconference from May 7th to 11th, meeting in the United States for the first Dear Members, time in twelve years, and for the very first The year 2002 ended with a lot of uncertainties. The economic recession has time in New York City. Complete registra- hit many people, created great doubts about investments and reduced tion information is available on the website, www.istm.org. Participants may consumption. It has also decreased air traffic, and many airlines are facing register on-line, by fax or by mail. Hotel serious identity and economic crises, forcing some of them to restructure. reservations for the conference at the International terrorism is certainly not dead, and we are facing a possible headquarters hotel, the Marriott Marquis, war in the Middle East. in the heart of Times Square, may also be Is economic globalization to blame? Is the economy ruling the world made now. allowing no room for a sound social and political counterbalance to create The program includes not only discus- more long lasting and constructive development trends? Is our planet sions on classic travelers’ ailments such divided between good and bad nations? Can’t we find ways to increase as diarrhea, dengue, and yellow fever, but access to medicine for those who need it the most? will also look at more exotic issues. A ple- We in travel medicine know that globalization also brings good things. -

Ladies 100% Silk Blouse This Information Is Intended to Serve As a Guide- Line for Embroiderers
Front LADIES 100% SI L K BL OUSE RH20 Back A smooth, elegant silhouette is achieved by the finest fabric and details. • 100% silk 3.3 oz Care Instructions: • Chest darts Dry clean only. • Cuffed short-sleeve hems Cool iron reverse side as needed. • Slightly curved hem Finished Measurements in Inches Colors Size XS S M L XL XXL Plus 1X Plus 2X Black Chest 18.5 19.5 21 22.5 24.75 25.75 26.75 29.75 Bright Blue Body 23.25 23.87 24.25 25.12 25.75 26.5 27 27.75 Citron Sleeve 6.75 7.25 7.75 8 8.37 8.75 9 9.25 Jade Shoulder 15 15.5 16 17 18 18.75 19.5 22 Papaya Sweep 19.5 20.5 22 23.5 25.75 26.75 27.75 30.75 Winter White Chest measured 1” under arm hole. Body length measured from center back neck seam to hem. Please Note: Colors shown are approximate Sleeve measured from shoulder seam to hem. Shoulder width measured from arm hole to arm hole. and for reference only. For closest match see Sweep measured side seam to side seam. PMS colors, or for exact match, returnable samples and grommeted samples are available. Color Chart Colors Black Bright Blue Citron Jade Papaya Winter White Textile PMS Process Black 659C 7403C 656C 170C No Match General PMS 19-1102TC 16-4032TC 12-0825TC 15-5812TC 15-1435TC 11-0104TC Madeira Classic 1007 1143 1066 1047 1152 1003 Red House Embroidery Recommendations Provided by the Embroidery Trade Association RH19/RH20 – 100% Silk Herringbone Campshirt & Ladies 100% Silk Blouse This information is intended to serve as a guide- line for embroiderers. -

CAMO BPA2 Spec23jan2014
SPECIFICATION Title: Printing of Experimental Pattern on 50/50 Nylon/Cotton Ripstop and 500 Denier Textured Nylon (Cordura), Rayon/Para-Aramid/Nylon Ripstop (Defender M) 1. Background and Discussion: Natick Soldier Systems Center requires rapid printed fabrics for field/lab testing of camouflage patterns for use in woodland, transitional and arid environments that conform to visual, NIR and SWIR requirements. Standards will be established once optimization phase is completed from production trails. 2. Objective: To provide all necessary materials, equipment, and personnel to ink jet print camouflage printed fabric at a minimum of 5 linear yards up 100 linear yards, sixty-inch width, of a Woodland, Transitional and Arid pattern with NIR and SWIR properties. Patterns shall be camouflage printed on 50/50 Nylon/Cotton Ripstop fabric conforming to the physical properties detailed in specification MIL-DTL-44436A. To provide all necessary materials, equipment, and personnel to ink jet print camouflage printed fabric at a minimum of 5 linear yards up 100 linear yards, sixty-inch width, of a Woodland, a Transitional and an Arid pattern with NIR and SWIR properties. Patterns shall be printed on 500 denier Textured Nylon conforming to the physical properties detailed in specification MIL-DTL- 32439. To provide all necessary materials, equipment, and personnel to ink jet print camouflage printed fabric at a minimum of 5 linear yards up 100 linear yards, sixty-inch width, of a Woodland, a Transitional and an Arid pattern with NIR and SWIR properties. Patterns shall be printed on rayon/para-aramid/nylon ripstop fabric conforming to the physical properties detailed in GL- PD-07-12. -

Supporting Information for Adv
Copyright WILEY-VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2020. Supporting Information for Adv. Mater. Technol., DOI: 10.1002/admt.202000383 Smart Thermally Actuating Textiles Vanessa Sanchez, Christopher J. Payne, Daniel J. Preston, Jonathan T. Alvarez, James C. Weaver, Asli T. Atalay, Mustafa Boyvat, Daniel M. Vogt, Robert J. Wood, George M. Whitesides, and Conor J. Walsh* Copyright WILEY-VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2018. Supporting Information Smart Thermally Actuating Textiles Vanessa Sanchez†, Christopher J. Payne†, Daniel J. Preston, Jonathan T. Alvarez, James C. Weaver, Asli T. Atalay, Mustafa Boyvat, Daniel M. Vogt, Robert J. Wood, George M. Whitesides, and Conor J. Walsh* †Indicates equal contribution. *to whom correspondence should be addressed: [email protected] 1. Detailed Materials and Fabrication of STATs 1.1 Laser Fabrication Methods Material patterning for smart thermally actuating textiles (STATs) was done using vector cutting on a laser cutter. All laser cutting was performed using a 60-Watt CO2 laser (VLS 6.60, Universal Laser Systems) with the 2.0 lens. According to the manufacturer, this lens has a focal length of 50.8 mm, a focal spot size of approximately 0.13 mm, and a 2.54-mm depth of focus which define the width, w, of the zone of ablation. The width was measured to be approximately 200 µm with the system configuration in this work (Figure S1). Figure S1. (A) Schematic and (B) SEM image indicating zone of ablation and width. 1 1.2 Textile Heater Fabrication Joule heaters were incorporated on the interior of STATs. For the active heating material, woven silver-plated nylon (Shieldex Bremen Ripstop, V Technical Textiles Inc.) was adhered to a 5 μm double-sided adhesive (3M #82600 5 μm electronic double-sided tape, Bristol Tape Corporation). -

New Cocoon CI 2012.Pdf
Introduction Approach Core Values System Design focus points: > development of a toolkit to build up a common threat, a consistent design language for the whole brand presence. > a balance of informative and emotional elements to get the message and brand values across > material icons and color codes to make product distinctions clear > additional mood images on the packaging to give guidance for product use > reduction of material and information, focus on the essence > optimization of the existing header system to guarantee easy packaging in the factory, damage-free delivery and perfect presentation in the store. Quality aspects Cocoon Core Values These are the core values of the brand cocoon. Light Weight Compact Original Both, the quality aspects and the emotional aspects, should come across in the new brand design and packaging. Quality Fair Mobile Bed Emotional aspects Cocoon as shelter Traveler first Cocoon Packaging System Explanation of the design system on the example of black is travel sheet logo update material description black area contains the technical product description grey is mummy liner with icon and material color information, e.g. the very important a silk travel sheet. comparison chart SLEEP SACK OR SLEEPING BAG LINER EXTREMELY LIGHTWEIGHT AND ROOMY Use: 100% Silk • in hotels, youth hostels, 100 % Seide huts and on boats Features: 100 % Soie ® 160 g • side opening with Velcro closure 5.6 oz. what is it? 86” x 35” 100 % Seda • pocket for pillow insert 220 x 90 cm 100 % • completely double-stitched with reinforced gussets LEICHT-REISESCHLAFSACK ODER INNENSCHLAFSACK EXTREM LEICHT UND GERÄUMIG 100% size weight Verwendung: Travel • in Hotels, Jugendherbergen, TM silk auf Berghütten und Booten 220 x 90 cm 160 g TM Ausführung: Travel Sheet premium quality 86” x 35” 5.6 oz. -

Repair Place Is Dave Page Cob- My Go-To Quick-Fix Solution Is …Duct Tape! Bler in Seattle’S Fremont Neighborhood
34 » Backcountry March + April 2011 » Washington Trails How to Do It » Fix It First A little elbow grease—and duct tape—will go a long way, and you can always call the professionals when the damage is serious As an avid hiker and climber, I tear through can also fix any Gore-Tex garments, backpacks, gear and garments at steady clip. I’ve found and any type of sleeping bag or insulated that the most cost effective way to keep me in jacket. If you’re terrified of throwing out your useful clothing and reliable gear is to mend my twenty-year-old boots or embarrassed to be damaged items rather than replacing them. seen breaking in new ones, one longtime local favorite boot repair place is Dave Page Cob- My go-to quick-fix solution is …duct tape! bler in Seattle’s Fremont neighborhood. In Port- No big surprise there, and yet I keep finding land, try Mountain Soles for boot repair. And new ways to use duct tape on my gear. Gaiters if you are looking to get your rock climbing sliced with a cram- shoes resoled, I recommend Cascade Cobblers pon? A rip in the in Winthrop. You can mail them your thrashed bottom of Gore-Tex shoes and they will send back grippy, beauti- pants after a glis- fully resoled shoes for a good price. sade? A tent pole stay meltdown? All And if the problem isn’t damaged gear, but this and more can no gear at all—try making it yourself! easily and quickly be mended with this If your skills go beyond duct tape and Seam silver wonder tape— Grip, stop by Seattle Fabrics. -

Dazian.Com Dazian Design Services the ELEMENTS OF
The Elements of Creativity www.dazian.com Dazian Design Services THE ELEMENTS OF DAZIAN PRODUCTS CREATIVITY Dz LED Scenic Elements Rental Dazian Products and Lighting Products Decor Since 1842, creativity has been the basic element of everything we do at Dazian. Today, we continue to make dreams materialize in the most dramatic ways, with unique fabric environments that Fabric Structures win applause the world over. An integrated fabric décor company with a network of innovative global resources, Dazian is your one‑ stop resource for expertise in FR Fabrics, Design, Custom Sewing, Custom Printing, Fabric Structures and Rentals. We work closely with designers to take their vision from inspiration to dazzling instal‑ lations. And we’re proud to say that at every stage, we have great chemistry with our customers. Projection Surfaces CALIFORNIA FLORIDA Tel: 877-432-9426 Tel: 888-318-2904 2 Fax: 818-287-3810 (8 AM to 5 PM PST) Fax: 407-219-9679 (9 AM to 5 PM EST) email: [email protected] email: [email protected] Decor Products and Accessories DreamWorld DAZIAN PRODUCTS Large Format Backdrops Digital Printing Theatrical Fabrics YOUR Specialty Products ONE STOP RESOURCE for every aspect of design and Production Services production Visit our Website for more details Custom Decor www.dazian.com Masking NEW JERSEY Drapery Tel: 877-232-9426 3 Fax: 201-549-1055 (9 AM ‑ 5 PM EST) email: [email protected] Dazian THE ELEMENTS OF EXTRAORDINARY DAZIAN MARKETS Ma Dazian Markets Across the full spectrum of markets, Dazian is your one‑stop resource for expertise in creating the extraordinary. -

The Airtime Issue
SUMMER 2012 THE AIRTIME ISSUE Helly Hansen catwalk Scandinavian Design is the cornerstone in all Helly Hansen gear. The optimal combination of purposeful design, protection and style. This is why professional mountain guides, patrollers and discerning enthusiasts choose Helly Hansen. cOnFIDent wHen It MatteRs Helly HanSen WeSTin ReSoRT Helly HanSen WHiSTleR Village STRoll Helly HanSen gRanVille 115-4090 WHiSTleR Way 108-4295 BlaCkComB Way 766 gRanVille STReeT WHiSTleR BC (604)932-0142 WHiSTleR BC VanCouVeR (604) 609-3932 DAILY BEAR VIEWING TOURS BEARS2 Hour Tours $119 Guests Under 16yrs $89 Private & Semi-Private Bear Viewing Eco-Tours Toll Free Enjoy one of the premier black bear viewing spots in Whistler. 1.888.501.4845 James Fougere, a local naturalist and wildlife photographer, will Local 1.604.966.7385 guide you on your wilderness black bear safari. Starti ng in May, black bears emerge from hibernati on and begin to feed on the new spring growth. www.WhistlerDiscoveryTours.com [email protected] This adventure will leave you appreciati ve of our natural world. Avid photographers will fi nd this a prime opportunity to capture stunning images! WHISTLER LAND ROVER EXCURSIONS Visit whistlerdiscoverytours.com for more Eco-Tours. : DANIELLE BALIK RIDER: PAT MULROONEY : DANIELLE BALIK RIDER: PAT FOR THOSE WHO KNOW NO BOUNDS. Adventures shouldn’t have boundaries and neither should your equipment. With 140mm of go-anywhere, ride-anything A.R.T. travel giving it equal prowess on the way up and on the way down, the Sight always delivers a ride without limits. norco.com johnhenrybikes.com riderepublic.com fanatykco.com 100-400 Brooksbank Ave, North Vancouver 1- 41340 Government Road, Squamish Unit # 6 - 4433 Sundial Place, Whistler 604.986.5534 604.898.1953 604.938.9455 UNLIMITED ADVENTURES.