The Polyphenolic Composition and Antioxidant Capacity of Yellow European Plums (Prunus Domestica L.) and Novel Golden Prunes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Comparative Taxonomic Study of Seeds of Some Plants of Rosaceae Family in Iraq
1 Plant Archives Vol. 20, Supplement 2, 2020 pp. 589-595 e-ISSN:2581-6063 (online), ISSN:0972-5210 A COMPARATIVE TAXONOMIC STUDY OF SEEDS OF SOME PLANTS OF ROSACEAE FAMILY IN IRAQ Zainab Abid Aun Ali Department of Biology, College of science for women, University of Baghdad, Iraq Abstract Seeds of 12 types of fruits of plants within the family Rosaceae, representing seven genera cultivated in Iraq were examined. The study included Pyrus communis L., Eriobotrya japonica (Thunb.) Lindl., Cydonia oblonga Mill., Crataegus azarulus L., Malus domestica Borkh., Prunus persica (L.) Batsch., Prunus persica var. nectarina (Sol.) Maxim., Prunus persica var. platycarpa (Decne.) L.H. Bailey, Prunus domestica L., Prunus domestica var. italica (Borkh.) Schneid., Prunus cerasus L ., Prunus armaniaca L. and Rosa damascene Mill. Seeds were glabrous except of R. damascene were pubescence. Seeds of Prunus were coated by brown thin dry cover (testa) and in other genera were near this color either reddish brown as of M. domestica or light brown as of C. azarulus or dark brown– black as of E. japonica . Most seeds were ovate or obovate but were elliptic in P. armaniaca , puffed Oblong in E. japonica , spherical in P. persica var. platycarpa and were hemi spherical in C. azarulus . Seeds of Prunus were covered by hard woody endocarp; each type has different color and surface sculptures. There were variations in the surface configurations of endocarps and seeds between genera; it can be used as taxonomic evidences in separating the nearest taxa. Keywords : Rosaceae, Pyrus , Eriobotrya , Cydonia , Crataegus , Malus. Prunus, Rosa Introduction have five petals (simple flowers), whereas high numbers of Rosaceae is cosmopolitan, 115 genera and 3200 petals (double flowers) are typical attributes of most of the species, with economic importance especially in the tropical cultivated roses (Annick Dobois et al., 2010). -

Plant List for VC54, North Lincolnshire
Plant List for Vice-county 54, North Lincolnshire 3 Vc61 SE TA 2 Vc63 1 SE TA SK NORTH LINCOLNSHIRE TF 9 8 Vc54 Vc56 7 6 5 Vc53 4 3 SK TF 6 7 8 9 1 2 3 4 5 6 Paul Kirby, 31/01/2017 Plant list for Vice-county 54, North Lincolnshire CONTENTS Introduction Page 1 - 50 Main Table 51 - 64 Summary Tables Red Listed taxa recorded between 2000 & 2017 51 Table 2 Threatened: Critically Endangered & Endangered 52 Table 3 Threatened: Vulnerable 53 Table 4 Near Threatened Nationally Rare & Scarce taxa recorded between 2000 & 2017 54 Table 5 Rare 55 - 56 Table 6 Scarce Vc54 Rare & Scarce taxa recorded between 2000 & 2017 57 - 59 Table 7 Rare 60 - 61 Table 8 Scarce Natives & Archaeophytes extinct & thought to be extinct in Vc54 62 - 64 Table 9 Extinct Plant list for Vice-county 54, North Lincolnshire The main table details all the Vascular Plant & Stonewort taxa with records on the MapMate botanical database for Vc54 at the end of January 2017. The table comprises: Column 1 Taxon and Authority 2 Common Name 3 Total number of records for the taxon on the database at 31/01/2017 4 Year of first record 5 Year of latest record 6 Number of hectads with records before 1/01/2000 7 Number of hectads with records between 1/01/2000 & 31/01/2017 8 Number of tetrads with records between 1/01/2000 & 31/01/2017 9 Comment & Conservation status of the taxon in Vc54 10 Conservation status of the taxon in the UK A hectad is a 10km. -

Fruit Trees, Nut Trees, Plants, Seeds, Books & Sundries
Agroforestry Research Trust Fruit trees, nut trees, plants, seeds, books & sundries www.agroforestry.co.uk Agroforestry: what is it? The simplest definition of agroforestry is that it is the integration of trees and agriculture/horticulture to create a more diverse growing system. In agroforestry the aim is to promote more use of perennial crops, notably tree and shrub crops, because they are more resilient, more sustainable and ecologically sound. Agroforestry systems can vary in complexity from the very simple, eg occasional trees planted in pastures to provide shade, emergency forage and nitrogen (via nitrogen- fixing bacteria), to the more complex systems like forest gardens, which may utilise hundreds of species to create a self-sustaining and interconnected system. The Agroforestry Research Trust The Trust is a registered charity, set up to educate and research into perennial crops and agroforestry. We have 20 acres of trial grounds in Devon including forest gardens and other systems. There are public guided tours through the growing season of our sites (see website at https://www.agroforestry.co.uk/research-site-tours/ for info and dates). We also run courses and seminars in forest gardening, growing nut crops and the forest garden greenhouse (with subtropical forest garden) in Devon. Dates and more info on website at https://www.agroforestry.co.uk/product-category/courses/. The Forest Garden Network This is an informal network of people planning or already cultivating their own forest gardens or agroforestry systems on farms, with the aim to facilitate visits to each other’s sites – one of the best ways to improve our knowledge about temperate agroforestry. -

500 College Road East, 201W Princeton, Nj 08540
TITLE PAGE: VOLUME 1 OF 1 CROP GROUPING PETITION – STONE FRUIT GROUP 12, TECHNICAL AMENDMENT TO 40 CFR 180.41 (c) (12) AND 180.1 (h) (Apricot; Apricot, Japanese; Capulin; Cherry, black; Cherry, Nanking; Cherry, sweet; Cherry, tart; Choke cherry; Jujube; Klamath plum; Nectarine; Peach; Plum; Plum, American; Plum, beach; Plum, cherry; Plum, Chickasaw; Plum, Damson; Plum, Japanese; Plumcot; Prune (fresh); Sloe) AUTHOR HONG CHEN, Ph.D. INTERREGIONAL RESEARCH PROJECT NO. 4 RUTGERS UNIVERSITY 500 COLLEGE ROAD EAST, 201W PRINCETON, NJ 08540 New Jersey Agricultural Experiment Station Publication No. A - 27200-01-07, Supported by State, U.S. Hatch Act and other U.S. Department of Agriculture funds. IR-4 Stone Fruit Crop Group Petition_PR#09896 Page 1 of 304 TABLE OF CONTENTS PAGE TITLE PAGE: VOLUME 1 OF 1 ....................................................................................................1 TABLE OF CONTENTS .................................................................................................................2 TECHNICAL AMENDMENT: .......................................................................................................3 PROPOSED AMENDMENT ......................................................................................... 3 BACKGROUND ............................................................................................................ 4 CULTURAL INFORMATION ...................................................................................... 5 PEST PROBLEMS ........................................................................................................ -

Virus Diseases and Noninfectious Disorders of Stone Fruits in North America
/ VIRUS DISEASES AND NONINFECTIOUS DISORDERS OF STONE FRUITS IN NORTH AMERICA Agriculture Handbook No. 437 Agricultural Research Service UNITED STATES DEPARTMENT OF AGRICULTURE VIRUS DISEASES AND NONINFECTIOUS DISORDERS OF STONE FRUITS IN NORTH AMERICA Agriculture Handbook No. 437 This handbook supersedes Agriculture Handbook 10, Virus Diseases and Other Disorders with Viruslike Symptoms of Stone Fruits in North America. Agricultural Research Service UNITED STATES DEPARTMENT OF AGRICULTURE Washington, D.C. ISSUED JANUARY 1976 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C 20402 — Price $7.10 (Paper Cover) Stock Number 0100-02691 FOREWORD The study of fruit tree virus diseases is a tedious process because of the time needed to produce experimental woody plants and, often, the long interval from inoculation until the development of diagnostic symptoms. The need for cooperation and interchange of information among investigators of these diseases has been apparent for a long time. As early as 1941, a conference was called by Director V. R. Gardner at Michigan State University to discuss the problem. One result of this early conference was the selection of a committee (E. M. Hildebrand, G. H. Berkeley, and D. Cation) to collect and classify both published and unpublished data on the nomenclature, symptoms, host range, geographical distribution, and other pertinent information on stone fruit virus diseases. This information was used to prepare a "Handbook of Stone Fruit Virus Diseases in North America," which was published in 1942 as a mis- cellaneous publication of the Michigan Agricultural Experiment Station. At a second conference of stone fruit virus disease workers held in Cleveland, Ohio, in 1944 under the chairmanship of Director Gardner, a Publication Committee (D. -

Lignocellulosic Feedstock Catalogue
Deliverable D72 Catalogue and data of lignocellulosic feedstock best candidates for the production of ethanol Grant agreement n°: 227498 Project acronym: BABETHANOL Project title: New feedstock and innovative transformation process for a more sustainable development and production of lignocellulosic ethanol. Funding Scheme: collaborative project for specific international cooperation actions SICA Due date of deliverable: M34 Actual submission date: M48 Start of the project: 01/05/2009 Duration of the project: 48 months Contribution from partners: IICA PROCISUR, UNIUD-DISA, UCR Lead partner: IICA PROCISUR Version n°: 1.0 Project co-funded by the European Commission within FP7 Dissemination level PP Public X RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) Page 1 of 209 Table of contents 1. Introduction .......................................................................................................... 5 2. Material and methods .......................................................................................... 6 2.1 STAGES FOR SCREENING THE FEEDSTOCK ..................................................................... 6 2.2 BIOMASS WASTE SUPPLY ........................................................................................... 6 2.3 WOOD BIOMASS WASTE DEMAND IN SOUTH AMERICA ..................................................... 12 2.4 CHEMICAL CHARACTERIZATION AND SCREENING ........................................................... -
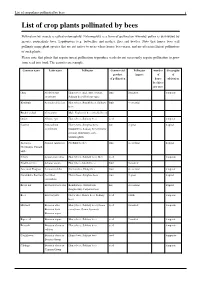
List of Crop Plants Pollinated by Bees 1 List of Crop Plants Pollinated by Bees
List of crop plants pollinated by bees 1 List of crop plants pollinated by bees Pollination by insects is called entomophily. Entomophily is a form of pollination whereby pollen is distributed by insects, particularly bees, Lepidoptera (e.g. butterflies and moths), flies and beetles. Note that honey bees will pollinate many plant species that are not native to areas where honey bees occur, and are often inefficient pollinators of such plants. Please note that plants that require insect pollination to produce seeds do not necessarily require pollination to grow from seed into food. The carrot is an example. Common name Latin name Pollinator Commercial Pollinator number Geography product impact of of of pollination honey cultivation bee hives per acre Okra Abelmoschus Honey bees (incl. Apis cerana), fruit 2-modest temperate esculentus Solitary bees (Halictus spp.) Kiwifruit Actinidia deliciosa Honey bees, Bumblebees, Solitary fruit 4-essential bees Bucket orchid Coryanthes Male Euglossini bees (Orchid bees) Onion Allium cepa Honey bees, Solitary bees seed temperate Cashew Anacardium Honey bees, Stingless bees, nut 3-great tropical occidentale bumblebees, Solitary bees (Centris tarsata), Butterflies, flies, hummingbirds Atemoya, Annona squamosa Nitidulid beetles fruit 4-essential tropical Cherimoya, Custard apple Celery Apium graveolens Honey bees, Solitary bees, flies seed temperate Strawberry tree Arbutus unedo Honey bees, bumblebees fruit 2-modest American Pawpaw Asimina triloba Carrion flies, Dung flies fruit 4-essential temperate Carambola, -

Pm4-030-1-En.Pdf
EPPO Standards SCHEMES FOR THE PRODUCTION OF HEALTHY PLANTS FOR PLANTING CERTIFICATION SCHEME FOR ALMOND, APRICOT, PEACH AND PLUM PM 4/30(1) English oepp eppo European and Mediterranean Plant Protection Organization 1, rue Le Nôtre, 75016 Paris, France 1 APPROVAL EPPO Standards are approved by EPPO Council. The date of approval appears in each individual standard. REVIEW EPPO Standards are subject to periodic review and amendment. The next review date for this set of EPPO Standards is decided by the EPPO Working Party on Phytosanitary Regulations. AMENDMENT RECORD Amendments will be issued as necessary, numbered and dated. The dates of amendment appear in each individual standard (as appropriate). DISTRIBUTION EPPO Standards are distributed by the EPPO Secretariat to all EPPO Member Governments. Copies are available to any interested person under particular conditions upon request to the EPPO Secretariat. SCOPE EPPO Schemes for the Production of Healthy Plants for Planting are intended to be used by NPPOs or equivalent authorities, in their capacity as bodies responsible for the design of systems for the production of healthy plants for planting, for the inspection of such plants proposed for certification, and for the issue of appropriate certificates. REFERENCES OEPP/EPPO (1991) Recommendations made by EPPO Council in 1990: general scheme for the production of certified pathogen-tested vegetatively propagated ornamental plants. Bulletin OEPP/EPPO Bulletin 21, 757. OEPP/EPPO (1992) Recommendations made by EPPO Council in 1981: certification of virus-tested fruit trees, scions and rootstocks. EPPO Technical Documents 1013, 42-43. OEPP/EPPO (1993) Recommendations made by EPPO Council in 1992: scheme for the production of classified vegetatively propagated ornamental plants to satisfy health standards. -

Tranzschelia Discolor (Fuckel) Tranzschel & Litv., 1939
-- CALIFORNIA D EPAUMENT OF cdfa FOOD & AGRICULTURE ~ California Pest Rating Proposal for Tranzschelia discolor (Fuckel) Tranzschel & Litv., 1939 Synonym Tranzschelia pruni-spinosae var. discolor (Fuckel) Dunegan, 1938 Stone fruit rust Domain: Eukaryota; Kingdom: Fungi; Phylum: Basidiomycota; Subphylum: Pucciniomycotina; Class: Pucciniomycetes; Order: Pucciniales Family: Uropyxidaceae Current Pest Rating: C Proposed Pest Rating: C Comment Period: 12/07/2020 through 01/21/2021 Initiating Event: A request for clarification on the rating for Tranzschelia discolor came from a County plant pathologist. The risk to California from T. discolor is described herein and a permanent rating is proposed. History & Status: Background: Tranzschelia discolor is a rust pathogen, mainly attacking plants in the genus Prunus. Almond, peach, and prune are the most susceptible hosts in California, but it also affects apricot, cherry, nectarine, and plum. It is a macrocyclic, heteroecious rust with multiple spore stages that develop on two different hosts. However, the alternate host is not necessary for epidemics to develop on Prunus. Prunus is the primary host and produces stage II (urediniospores), stage III (teliospores), and stage IV (basidiospores). The alternate host is Anemone coronaria which produces stage 0 (spermatia) and stage 1 (aeciospores). Urediniospores are the repeating, vegetative spores and can autoinfect their Prunus host. The pathogen can be separated into formae speciales (f. sp.), based on host specificity in the uredinial infection stage. Bolkan et al., 1985, showed that the uredinial stages are specific to the -- CALIFORNIA D EPAUMENT OF cdfa FOOD & AGRICULTURE ~ host where they are produced and do not cross-infect. These forms are T. discolor f. sp. -

1 14 / 19741 EXTRAPOLATION TABLE for EFFECTIVENESS Of
14 / 19741 EXTRAPOLATION TABLE for EFFECTIVENESS of HERBICIDES : ►WEEDS IN STONE FRUIT INTRODUCTION The table provides detailed lists of acceptable extrapolations organized by crop groups, for regulatory authorities and applicants, in the context of the registration of plant protection products for minor uses. The table should be used in conjunction with the EPPO Standard PP1/257- Efficacy and crop safety extrapolations for minor uses. It is important to ensure that expert judgment and regulatory experience are employed when using these tables. EPPO excludes liability as to the reliability of the information provided through these tables. The scope for extrapolation may be extended as data and experience with a certain plant protection products increases. The applicant should always provide appropriate justification and information to support the proposed extrapolation. For example, comparability of target biology may be a relevant factor, either in extrapolating to other target species or for the same target onto another crop. For crops, factors such as comparable growth habit, structure etc. should be considered. TABLE FORMAT The main pest species for the crop group are listed in Column 1 (although this is not exhaustive), and the pest group to which they belong is specified in Column 2. Companies may choose if they wish to provide data only for individual named species, which would then appear individually listed on the label. But underlined species have been identified as key major targets and as such it is advisable to generate data on these. Furthermore, data on these species then allow a claim to be made for the whole pest group (as specified in Column 2), if required. -

SUBSIDIARY LEGISLATION 549.123 TREES and WOODLANDS PROTECTION REGULATIONS 31St July, 2018
TREES AND WOODLANDS PROTECTION [S.L.549.123 1 SUBSIDIARY LEGISLATION 549.123 TREES AND WOODLANDS PROTECTION REGULATIONS 31st July, 2018 LEGAL NOTICE 258 of 2018. 1. (1) The title of these regulations is the Trees and Citation and Woodlands Protection Regulations. commencement. (2) Regulation 19 shall come into force on such a date as the Minister responsible for the Environment may by notice in the Gazette declare. 2. The objectives of these regulations are to protect trees Scope. and woodlands in the Maltese Islands, safeguard them, and to regulate the activities that may have an effect thereon. PART I: INTERPRETATION AND ADMINISTRATION 3. (1) In these regulations, unless the context otherwise Interpretation. requires: "Act" means the Environment Protection Act; Cap. 549. "agricultural land" means arable land that is lawfully used for cultivation, and excludes any non-arable land or wild trees even if located within the confines of an agricultural holding; "Authority" or "Competent Authority" means the Environment and Resources Authority as established in article 6 of the Act; "compensatory planting" means the planting or provision of one or more trees, as a replacement or compensation for other trees that have been, or will be, uprooted, felled, damaged or removed; "director" means the director responsible for environment protection within the Authority or any person delegated or commissioned by the Director or otherwise acting lawfully on his behalf; "director responsible for plant health" means the director as defined in the Plant -

An Identification Guide to Berries
An identification guide to berries A quick A-Z guide to identifying common berries for the BTO Winter Thrushes Survey. Below are some hints to help you identify the berries that wintering thrushes may be feeding on. Later in the season leaves may not be present on some of these species, making identification more challenging. This is not meant as a definitive guide, and there are many other species of berry-bearing plant found in the countryside. If you are in any doubt about the identification of a berry-bearing plant, we recommend you refer to a fieldguide, or you could look at pictures of these species on the internet (try typing the plant name into Google, for instance.) Bramble (Blackberry) Blackthorn (Sloe) Rubus fruticosus agg. Prunus spinosa The familiar blackberry Marble-sized, deep fruit, ingredient in so purple, bitter fruits with a many pies. Found on distinctive white ‘bloom’ low-growing, thorny briars on the skin, sometimes which twine aggressively used to make sloe gin. through hedges, woodland Very thorny common and gardens. hedgerow shrub or tree. Buckthorn Cotoneaster Rhamnus catharticus Cotoneaster sp. A common, spined, A very frequent garden hedgerow plant, with shrub, occasional escape distinctive leaf veins that and rare wild plant. curve in towards the mid- Abundant unstalked, small rib. Small black berries are berries and, usually, small arranged in whorls around leaves on spineless twigs. and along the branches. Compact shrub or tree. Dogwood Elder (Elderberry) Cornus sanguinea Sambucus nigra A common chalk soil Frequent small tree, shrub. Highly distinctive red generally on calcareous soils.