Post-Larval Developm Ent and Sexual Dim Orphism of the Spider Crab M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Ecosystem Model of the North Sea to Support an Ecosystem Approach to Fisheries Management: Description and Parameterisation
Science Series Technical Report no.142 An ecosystem model of the North Sea to support an ecosystem approach to fisheries management: description and parameterisation S. Mackinson and G. Daskalov Science Series Technical Report no.142 An ecosystem model of the North Sea to support an ecosystem approach to fisheries management: description and parameterisation S. Mackinson and G. Daskalov This report should be cited as: Mackinson, S. and Daskalov, G., 2007. An ecosystem model of the North Sea to support an ecosystem approach to fisheries management: description and parameterisation. Sci. Ser. Tech Rep., Cefas Lowestoft, 142: 196pp. This report represents the views and findings of the authors and not necessarily those of the funders. © Crown copyright, 2008 This publication (excluding the logos) may be re-used free of charge in any format or medium for research for non-commercial purposes, private study or for internal circulation within an organisation. This is subject to it being re-used accurately and not used in a misleading context. The material must be acknowledged as Crown copyright and the title of the publication specified. This publication is also available at www.Cefas.co.uk For any other use of this material please apply for a Click-Use Licence for core material at www.hmso.gov.uk/copyright/licences/ core/core_licence.htm, or by writing to: HMSO’s Licensing Division St Clements House 2–16 Colegate Norwich NR3 1BQ Fax: 01603 723000 E-mail: [email protected] List of contributors and reviewers Name Affiliation -

Part I. an Annotated Checklist of Extant Brachyuran Crabs of the World
THE RAFFLES BULLETIN OF ZOOLOGY 2008 17: 1–286 Date of Publication: 31 Jan.2008 © National University of Singapore SYSTEMA BRACHYURORUM: PART I. AN ANNOTATED CHECKLIST OF EXTANT BRACHYURAN CRABS OF THE WORLD Peter K. L. Ng Raffles Museum of Biodiversity Research, Department of Biological Sciences, National University of Singapore, Kent Ridge, Singapore 119260, Republic of Singapore Email: [email protected] Danièle Guinot Muséum national d'Histoire naturelle, Département Milieux et peuplements aquatiques, 61 rue Buffon, 75005 Paris, France Email: [email protected] Peter J. F. Davie Queensland Museum, PO Box 3300, South Brisbane, Queensland, Australia Email: [email protected] ABSTRACT. – An annotated checklist of the extant brachyuran crabs of the world is presented for the first time. Over 10,500 names are treated including 6,793 valid species and subspecies (with 1,907 primary synonyms), 1,271 genera and subgenera (with 393 primary synonyms), 93 families and 38 superfamilies. Nomenclatural and taxonomic problems are reviewed in detail, and many resolved. Detailed notes and references are provided where necessary. The constitution of a large number of families and superfamilies is discussed in detail, with the positions of some taxa rearranged in an attempt to form a stable base for future taxonomic studies. This is the first time the nomenclature of any large group of decapod crustaceans has been examined in such detail. KEY WORDS. – Annotated checklist, crabs of the world, Brachyura, systematics, nomenclature. CONTENTS Preamble .................................................................................. 3 Family Cymonomidae .......................................... 32 Caveats and acknowledgements ............................................... 5 Family Phyllotymolinidae .................................... 32 Introduction .............................................................................. 6 Superfamily DROMIOIDEA ..................................... 33 The higher classification of the Brachyura ........................ -

OREGON ESTUARINE INVERTEBRATES an Illustrated Guide to the Common and Important Invertebrate Animals
OREGON ESTUARINE INVERTEBRATES An Illustrated Guide to the Common and Important Invertebrate Animals By Paul Rudy, Jr. Lynn Hay Rudy Oregon Institute of Marine Biology University of Oregon Charleston, Oregon 97420 Contract No. 79-111 Project Officer Jay F. Watson U.S. Fish and Wildlife Service 500 N.E. Multnomah Street Portland, Oregon 97232 Performed for National Coastal Ecosystems Team Office of Biological Services Fish and Wildlife Service U.S. Department of Interior Washington, D.C. 20240 Table of Contents Introduction CNIDARIA Hydrozoa Aequorea aequorea ................................................................ 6 Obelia longissima .................................................................. 8 Polyorchis penicillatus 10 Tubularia crocea ................................................................. 12 Anthozoa Anthopleura artemisia ................................. 14 Anthopleura elegantissima .................................................. 16 Haliplanella luciae .................................................................. 18 Nematostella vectensis ......................................................... 20 Metridium senile .................................................................... 22 NEMERTEA Amphiporus imparispinosus ................................................ 24 Carinoma mutabilis ................................................................ 26 Cerebratulus californiensis .................................................. 28 Lineus ruber ......................................................................... -

Symbiotic Brachyura1)
CHAPTER 71-10 SYMBIOTIC BRACHYURA1) BY PETER CASTRO Contents. – Introduction – The meaning of symbiosis – Categories of symbiosis. Cryptochi- roidea: Cryptochiridae – Hosts, biogeography, and ecology – Life history – Nutrition. Pilum- noidea: Pilumnidae – Hosts, biogeography, and ecology – Life history – Nutrition. Pinnotheroidea: Aphanodactylidae and Pinnotheridae – Hosts, biogeography, and ecology – Life history – Nutri- tion. Trapezioidea: Domeciidae – Hosts, biogeography, and ecology. Trapezioidea: Tetraliidae – Hosts, biogeography, and ecology – Life history – Nutrition. Trapezioidea: Trapeziidae –Hosts, biogeography, and ecology – Life history – Nutrition. Other Brachyura – Majoidea – Portunoidea – Xanthoidea – Other Xanthoidea – Miscellaneous groups of Brachyura. Acknowledgements. Ap- pendix. Bibliography. INTRODUCTION Close heterospecific associations are ubiquitous in most if not all biotic communi- ties. These associations vary widely in character, and can include a wide range of mor- phological, ecological, physiological, and/or behavioural adaptations, at times the result of coevolution between the partners. Brachyuran crabs are common participants in such asso- ciations, e.g., as hosts for internal or external parasites (see Chapter 71-12 in this volume), in associations with other organisms for concealment (see Chapter 71-11 in this volume), and as close associates living on or within an invertebrate host (the symbiotic associations discussed in this chapter). The variety and range of complexity among these symbioses is remarkable and difficult to categorize. Brachyuran associates are often referred to as “commensals”, “parasites”, “mutualists”, or, as herein, simply as “symbionts”. The groups of symbiotic brachyurans discussed in this chapter are listed alphabetically by superfamilies, which follow the classification in Chapter 71-18 in this volume. Various 1) Manuscript concluded October 2014; final additions May 2015. © Koninklijke Brill NV, Leiden, 2015 Crustacea 9C (71-10): 543-581 544 P. -

For Review Only 19 20 21 504 Ampuero D, T
Page 1 of 39 Zoological Journal of the Linnean Society 1 2 3 1 DNA identification and larval morphology provide new evidence on the systematic 4 5 2 position of Ergasticus clouei A. Milne-Edwards, 1882 (Decapoda, Brachyura, 6 7 3 Majoidea) 8 9 10 4 11 1 2 1 3 12 5 Marco-Herrero, Elena , Torres, Asvin P. , Cuesta, José A. , Guerao, Guillermo , Palero, 13 14 6 Ferran 4, & Abelló, Pere 5 15 16 7 17 18 8 1Instituto de CienciasFor Marinas Review de Andalucía (ICMAN-C OnlySIC), Avda. República 19 20 21 9 Saharaui, 2, 11519 Puerto Real, Cádiz, Spain. 22 2 23 10 Instituto Español de Oceanografía, Centre Oceanogràfic de les Balears, Moll de Ponent 24 25 11 s/n, 07015 Palma, Spain. 26 27 12 3IRTA, Unitat de Cultius Aqüàtics. Ctra. Poble Nou, Km 5.5, 43540 Sant Carles de la 28 29 30 13 Ràpita, Tarragona, Spain. 31 4 32 14 Unitat Mixta Genòmica i Salut CSISP-UV, Institut Cavanilles Universitat de Valencia, 33 34 15 C/ Catedrático José Beltrán 2, 46980 Paterna, Spain. 35 36 16 5Institut de Ciències del Mar (CSIC), Passeig Marítim de la Barceloneta 37-49, 08003 37 38 17 Barcelona, Catalonia. Spain. 39 40 41 18 42 43 19 44 45 20 46 47 21 RUN TITLE: Larval evidence and the systematic position of Ergasticus clouei 48 49 50 22 51 52 53 54 55 56 57 58 59 60 Zoological Journal of the Linnean Society Page 2 of 39 1 2 3 23 ABSTRACT: The morphology of the complete larval stage series of the crab Ergasticus 4 5 24 clouei is described and illustrated based on larvae (zoea I, zoea II and megalopa) 6 7 25 captured from plankton samples taken in Mediterranean waters. -
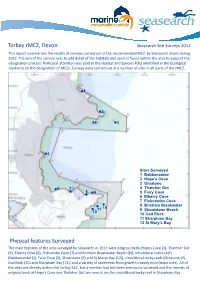
Torbay Rmcz, Devon Physical Features Surveyed
Torbay rMCZ, Devon Seasearch Site Surveys 2012 This report summarises the results of surveys carried out in the recommended MCZ by Seasearch divers during 2012. The aim of the surveys was to add detail of the habitats and species found within the area to support the designation process. Particular attention was paid to the Habitat and Species FOCI identified in the Ecological Guidance on the designation of MCZs. Surveys were carried out at a number of sites in all parts of the rMCZ. 1 2 4 3 5 Sites Surveyed 1 Babbacombe 2 Hope’s Cove CW 3 Orestone 6 7 4 Thatcher Gut 8 9 5 Fairy Cove 6 Elberry Cove 7 Fishcombe Cove 8 Brixham Breakwater 10 11 9 Shoalstone Beach 12 10 Cod Rock 11 Sharpham Bay 12 St Mary’s Bay Physical features Surveyed The main features of the area surveyed by Seasearch in 2012 were eelgrass beds (Hopes Cove (2), Thatcher Gut (4), Elberry Cove (6), Fishcombe Cove (7) and Brixham Breakwater Beach (8)), infralittoral rocky reefs (Babbacombe (1), Fairy Cove (5), Shoalstone (9) and St Marys Bay (12)), circalittoral rocky reefs (Orestone (3), Cod Rock (10) and Sharpham Bay (11)) and a variety of sediments from gravel to sandy mud (most sites). All of the sites are already within the Torbay SAC, but a number had not been previously surveyed and the records of eelgrass beds at Hope’s Cove and Thatcher Gut are new, is arCWe the circalittoral rocky reef in Sharpham Bay. CW Features of the Marine Life and Habitats Eeelgrass Beds (Sites 2,4,6,7,8) Eelgrass, Zostera marina, is one of the very few flowering plants underwater and, unlike seaweeds, has a system of roots which can help to bind soft sediments and create a complex habitat in what would otherwise be a relatively un-diverse environment. -

Decapoda, Brachyura
APLICACIÓN DE TÉCNICAS MORFOLÓGICAS Y MOLECULARES EN LA IDENTIFICACIÓN DE LA MEGALOPA de Decápodos Braquiuros de la Península Ibérica bérica I enínsula P raquiuros de la raquiuros B ecápodos D de APLICACIÓN DE TÉCNICAS MORFOLÓGICAS Y MOLECULARES EN LA IDENTIFICACIÓN DE LA MEGALOPA LA DE IDENTIFICACIÓN EN LA Y MOLECULARES MORFOLÓGICAS TÉCNICAS DE APLICACIÓN Herrero - MEGALOPA “big eyes” Leach 1793 Elena Marco Elena Marco-Herrero Programa de Doctorado en Biodiversidad y Biología Evolutiva Rd. 99/2011 Tesis Doctoral, Valencia 2015 Programa de Doctorado en Biodiversidad y Biología Evolutiva Rd. 99/2011 APLICACIÓN DE TÉCNICAS MORFOLÓGICAS Y MOLECULARES EN LA IDENTIFICACIÓN DE LA MEGALOPA DE DECÁPODOS BRAQUIUROS DE LA PENÍNSULA IBÉRICA TESIS DOCTORAL Elena Marco-Herrero Valencia, septiembre 2015 Directores José Antonio Cuesta Mariscal / Ferran Palero Pastor Tutor Álvaro Peña Cantero Als naninets AGRADECIMIENTOS-AGRAÏMENTS Colaboración y ayuda prestada por diferentes instituciones: - Ministerio de Ciencia e Innovación (actual Ministerio de Economía y Competitividad) por la concesión de una Beca de Formación de Personal Investigador FPI (BES-2010- 033297) en el marco del proyecto: Aplicación de técnicas morfológicas y moleculares en la identificación de estados larvarios planctónicos de decápodos braquiuros ibéricos (CGL2009-11225) - Departamento de Ecología y Gestión Costera del Instituto de Ciencias Marinas de Andalucía (ICMAN-CSIC) - Club Náutico del Puerto de Santa María - Centro Andaluz de Ciencias y Tecnologías Marinas (CACYTMAR) - Instituto Español de Oceanografía (IEO), Centros de Mallorca y Cádiz - Institut de Ciències del Mar (ICM-CSIC) de Barcelona - Institut de Recerca i Tecnología Agroalimentàries (IRTA) de Tarragona - Centre d’Estudis Avançats de Blanes (CEAB) de Girona - Universidad de Málaga - Natural History Museum of London - Stazione Zoologica Anton Dohrn di Napoli (SZN) - Universitat de Barcelona AGRAÏSC – AGRADEZCO En primer lugar quisiera agradecer a mis directores, el Dr. -

A History of the British Stalk-Eyed Crustacea
Go ygle Acerca de este libro Esta es una copia digital de un libro que, durante generaciones, se ha conservado en las estanterias de una biblioteca, hasta que Google ha decidido escanearlo como parte de un proyecto que pretende que sea posible descubrir en linea libros de todo el mundo. Ha sobrevivido tantos anos como para que los derechos de autor hayan expirado y el libro pase a ser de dominio publico. El que un libro sea de dominio publico significa que nunca ha estado protegido por derechos de autor, o bien que el periodo legal de estos derechos ya ha expirado. Es posible que una misma obra sea de dominio publico en unos paises y, sin embargo, no lo sea en otros. Los libros de dominio publico son nuestras puertas hacia el pasado, suponen un patrimonio historico, cultural y de conocimientos que, a menudo, resulta dificil de descubrir. Todas las anotaciones, marcas y otras senales en los margenes que esten presentes en el volumen original apareceran tambien en este archivo como testimonio del largo viaje que el libro ha recorrido desde el editor hasta la biblioteca y, finalmente, hasta usted. Normas de uso Google se enorgullece de poder colaborar con distintas bibliotecas para digitalizar los materiales de dominio publico a fin de hacerlos accesibles a todo el mundo. Los libros de dominio publico son patrimonio de todos, nosotros somos sus humildes guardianes. No obstante, se trata de un trabajo caro. Por este motivo, y para poder ofrecer este recurso, hemos tornado medidas para evitar que se produzca un abuso por parte de terceros con fines comerciales, y hemos incluido restricciones tecnicas sobre las solicitudes automatizadas. -

Larval Growth
LARVAL GROWTH Edited by ADRIAN M.WENNER University of California, Santa Barbara OFFPRINT A.A.BALKEMA/ROTTERDAM/BOSTON DARRYL L.FELDER* / JOEL W.MARTIN** / JOSEPH W.GOY* * Department of Biology, University of Louisiana, Lafayette, USA ** Department of Biological Science, Florida State University, Tallahassee, USA PATTERNS IN EARLY POSTLARVAL DEVELOPMENT OF DECAPODS ABSTRACT Early postlarval stages may differ from larval and adult phases of the life cycle in such characteristics as body size, morphology, molting frequency, growth rate, nutrient require ments, behavior, and habitat. Primarily by way of recent studies, information on these quaUties in early postlarvae has begun to accrue, information which has not been previously summarized. The change in form (metamorphosis) that occurs between larval and postlarval life is pronounced in some decapod groups but subtle in others. However, in almost all the Deca- poda, some ontogenetic changes in locomotion, feeding, and habitat coincide with meta morphosis and early postlarval growth. The postmetamorphic (first postlarval) stage, here in termed the decapodid, is often a particularly modified transitional stage; terms such as glaucothoe, puerulus, and megalopa have been applied to it. The postlarval stages that fol low the decapodid successively approach more closely the adult form. Morphogenesis of skeletal and other superficial features is particularly apparent at each molt, but histogenesis and organogenesis in early postlarvae is appreciable within intermolt periods. Except for the development of primary and secondary sexual organs, postmetamorphic change in internal anatomy is most pronounced in the first several postlarval instars, with the degree of anatomical reorganization and development decreasing in each of the later juvenile molts. -

ECOLOGIA POPULACIONAL DE Libinia Ferreirae (BRACHYURA: MAJOIDEA) NO LITORAL SUDESTE DO BRASIL
UNIVERSIDADE ESTADUAL PAULISTA INSTITUTO DE BIOCIÊNCIAS GESLAINE RAFAELA LEMOS GONÇALVES ECOLOGIA POPULACIONAL DE Libinia ferreirae (BRACHYURA: MAJOIDEA) NO LITORAL SUDESTE DO BRASIL DISSERTAÇÃO DE MESTRADO BOTUCATU 2016 UNIVERSIDADE ESTADUAL PAULISTA INSTITUTO DE BIOCIÊNCIAS DISSERTAÇÃO DE MESTRADO Ecologia populacional de Libinia ferreirae (Brachyura: Majoidea) no litoral sudeste do Brasil Geslaine Rafaela Lemos Gonçalves Orientador: Prof. Dr. Antonio Leão Castilho Coorientadora: Profª. Drª. Maria Lucia Negreiros Fransozo Dissertação apresentada ao Instituto de Biociências da Universidade Estadual Paulista “Júlio de Mesquita Filho” – UNESP – Câmpus Botucatu, como parte dos requisitos para obtenção do Título de Mestre em Ciências, curso de Pós-graduação em Ciências Biológicas, Área de Concentração: Zoologia. Botucatu – SP 2016 FICHA CATALOGRÁFICA ELABORADA PELA SEÇÃO TÉC. AQUIS. TRATAMENTO DA INFORM. DIVISÃO TÉCNICA DE BIBLIOTECA E DOCUMENTAÇÃO - CÂMPUS DE BOTUCATU - UNESP BIBLIOTECÁRIA RESPONSÁVEL: ROSEMEIRE APARECIDA VICENTE-CRB 8/5651 Gonçalves, Geslaine Rafaela Lemos. Ecologia populacional de Libinia ferreirae (Brachyura: Majoidea) no litoral sudeste do Brasil / Geslaine Rafaela Lemos Gonçalves. - Botucatu, 2016 Dissertação (mestrado) - Universidade Estadual Paulista "Júlio de Mesquita Filho", Instituto de Biociências de Botucatu Orientador: Antonio Leão Castilho Coorientador: Maria Lucia Negreiros Fransozo Capes: 20402007 1. Caranguejo. 2. Dinâmica populacional. 3. Hábitos alimentares. 4. Ecologia de populações. 5. Epizoísmo. Palavras-chave: Ciclo de vida; Crescimento; Dinâmica populacional; Epizoísmo; Hábitos alimentares. NEBECC Núcleo de Estudos em Biologia, Ecologia e Cultivo de Crustáceos II “Sou biólogo e viajo pela savana de meu país. Nessa região encontro gente que não sabe ler livros. Mas que sabe ler o mundo. Nesse universo de outros saberes, sou eu o analfabeto” Mia Couto “O saber a gente aprende com os mestres e com os livros. -

Edinburgh Research Explorer
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Edinburgh Research Explorer Edinburgh Research Explorer Some observations on the Mesolithic crustacean assemblage from Ulva Cave, Inner Hebrides, Scotland. Citation for published version: Pickard, C & Bonsall, C 2009, 'Some observations on the Mesolithic crustacean assemblage from Ulva Cave, Inner Hebrides, Scotland.'. in JM Burdukiewicz, K Cyrek, P Dyczek & K Szymczak (eds), Understanding the Past: Papers Offered to Stefan K. Kozowski. University of Warsaw Center for Research on the Antiquity of Southeastern Europe, Warsaw, pp. 305-313. Link: Link to publication record in Edinburgh Research Explorer Document Version: Publisher final version (usually the publisher pdf) Published In: Understanding the Past Publisher Rights Statement: © Pickard, C., & Bonsall, C. (2009). Some observations on the Mesolithic crustacean assemblage from Ulva Cave, Inner Hebrides, Scotland.In J. M. Burdukiewicz, K. Cyrek, P. Dyczek, & K. Szymczak (Eds.), Understanding the Past. (pp. 305-313). Warsaw: University of Warsaw Center for Research on the Antiquity of Southeastern Europe. General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. -

Hermit Crabs - Paguridae and Diogenidae
Identification Guide to Marine Invertebrates of Texas by Brenda Bowling Texas Parks and Wildlife Department April 12, 2019 Version 4 Page 1 Marine Crabs of Texas Mole crab Yellow box crab Giant hermit Surf hermit Lepidopa benedicti Calappa sulcata Petrochirus diogenes Isocheles wurdemanni Family Albuneidae Family Calappidae Family Diogenidae Family Diogenidae Blue-spot hermit Thinstripe hermit Blue land crab Flecked box crab Paguristes hummi Clibanarius vittatus Cardisoma guanhumi Hepatus pudibundus Family Diogenidae Family Diogenidae Family Gecarcinidae Family Hepatidae Calico box crab Puerto Rican sand crab False arrow crab Pink purse crab Hepatus epheliticus Emerita portoricensis Metoporhaphis calcarata Persephona crinita Family Hepatidae Family Hippidae Family Inachidae Family Leucosiidae Mottled purse crab Stone crab Red-jointed fiddler crab Atlantic ghost crab Persephona mediterranea Menippe adina Uca minax Ocypode quadrata Family Leucosiidae Family Menippidae Family Ocypodidae Family Ocypodidae Mudflat fiddler crab Spined fiddler crab Longwrist hermit Flatclaw hermit Uca rapax Uca spinicarpa Pagurus longicarpus Pagurus pollicaris Family Ocypodidae Family Ocypodidae Family Paguridae Family Paguridae Dimpled hermit Brown banded hermit Flatback mud crab Estuarine mud crab Pagurus impressus Pagurus annulipes Eurypanopeus depressus Rithropanopeus harrisii Family Paguridae Family Paguridae Family Panopeidae Family Panopeidae Page 2 Smooth mud crab Gulf grassflat crab Oystershell mud crab Saltmarsh mud crab Hexapanopeus angustifrons Dyspanopeus