An Ecosystem Model of the North Sea to Support an Ecosystem Approach to Fisheries Management: Description and Parameterisation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Illustrated Keys for the Identi¢Cation of the Pleocyemata (Crustacea: Decapoda) Zoeal Stages, from the Coastal Region of South-Western Europe
J. Mar. Biol. Ass. U.K. (2004), 84, 205^227 Printed in the United Kingdom Illustrated keys for the identi¢cation of the Pleocyemata (Crustacea: Decapoda) zoeal stages, from the coastal region of south-western Europe Antonina dos Santos*P and Juan Ignacio Gonza¤ lez-GordilloO *Instituto de Investigac° a‹ o das Pescas e do Mar, Avenida de Brasilia s/n, 1449-006 Lisbon, Portugal. OCentro Andaluz de Ciencia y Tecnolog|¤a Marinas, Universidad de Ca¤ diz, Campus de Puerto Real, 11510öPuerto Real (Ca¤ diz), Spain. PCorresponding author, e-mail: [email protected] The identi¢cation keys of the zoeal stages of Pleocyemata decapod larvae from the coastal region of south-western Europe, based on both new and previously published descriptions and illustrations, are provided. The keys cover 127 taxa, most of them identi¢ed to genus and species level. These keys were mainly constructed upon external morphological characters, which are easy to observe under a stereo- microscope. Moreover, the presentation of detailed ¢gures allows a non-specialist to make identi¢cations more easily. INTRODUCTION nearby areas as a complement document when identifying larval stages. Identi¢cation of decapod larvae from plankton samples The order Decapoda comprises two suborders, the is not easy, principally because there are great morpholo- Dendrobranchiata and the Pleocyemata (Martin & Davis, gical changes between developmental phases, although less 2001). A key for the identi¢cation of Dendrobranchiata pronounced between larval stages. Moreover, larval larvae covering the same area of this study has been descriptions of many species are still unsuitable or even presented by dos Santos & Lindley (2001). -

A Biotope Sensitivity Database to Underpin Delivery of the Habitats Directive and Biodiversity Action Plan in the Seas Around England and Scotland
English Nature Research Reports Number 499 A biotope sensitivity database to underpin delivery of the Habitats Directive and Biodiversity Action Plan in the seas around England and Scotland Harvey Tyler-Walters Keith Hiscock This report has been prepared by the Marine Biological Association of the UK (MBA) as part of the work being undertaken in the Marine Life Information Network (MarLIN). The report is part of a contract placed by English Nature, additionally supported by Scottish Natural Heritage, to assist in the provision of sensitivity information to underpin the implementation of the Habitats Directive and the UK Biodiversity Action Plan. The views expressed in the report are not necessarily those of the funding bodies. Any errors or omissions contained in this report are the responsibility of the MBA. February 2003 You may reproduce as many copies of this report as you like, provided such copies stipulate that copyright remains, jointly, with English Nature, Scottish Natural Heritage and the Marine Biological Association of the UK. ISSN 0967-876X © Joint copyright 2003 English Nature, Scottish Natural Heritage and the Marine Biological Association of the UK. Biotope sensitivity database Final report This report should be cited as: TYLER-WALTERS, H. & HISCOCK, K., 2003. A biotope sensitivity database to underpin delivery of the Habitats Directive and Biodiversity Action Plan in the seas around England and Scotland. Report to English Nature and Scottish Natural Heritage from the Marine Life Information Network (MarLIN). Plymouth: Marine Biological Association of the UK. [Final Report] 2 Biotope sensitivity database Final report Contents Foreword and acknowledgements.............................................................................................. 5 Executive summary .................................................................................................................... 7 1 Introduction to the project .............................................................................................. -

For Review Only 19 20 21 504 Ampuero D, T
Page 1 of 39 Zoological Journal of the Linnean Society 1 2 3 1 DNA identification and larval morphology provide new evidence on the systematic 4 5 2 position of Ergasticus clouei A. Milne-Edwards, 1882 (Decapoda, Brachyura, 6 7 3 Majoidea) 8 9 10 4 11 1 2 1 3 12 5 Marco-Herrero, Elena , Torres, Asvin P. , Cuesta, José A. , Guerao, Guillermo , Palero, 13 14 6 Ferran 4, & Abelló, Pere 5 15 16 7 17 18 8 1Instituto de CienciasFor Marinas Review de Andalucía (ICMAN-C OnlySIC), Avda. República 19 20 21 9 Saharaui, 2, 11519 Puerto Real, Cádiz, Spain. 22 2 23 10 Instituto Español de Oceanografía, Centre Oceanogràfic de les Balears, Moll de Ponent 24 25 11 s/n, 07015 Palma, Spain. 26 27 12 3IRTA, Unitat de Cultius Aqüàtics. Ctra. Poble Nou, Km 5.5, 43540 Sant Carles de la 28 29 30 13 Ràpita, Tarragona, Spain. 31 4 32 14 Unitat Mixta Genòmica i Salut CSISP-UV, Institut Cavanilles Universitat de Valencia, 33 34 15 C/ Catedrático José Beltrán 2, 46980 Paterna, Spain. 35 36 16 5Institut de Ciències del Mar (CSIC), Passeig Marítim de la Barceloneta 37-49, 08003 37 38 17 Barcelona, Catalonia. Spain. 39 40 41 18 42 43 19 44 45 20 46 47 21 RUN TITLE: Larval evidence and the systematic position of Ergasticus clouei 48 49 50 22 51 52 53 54 55 56 57 58 59 60 Zoological Journal of the Linnean Society Page 2 of 39 1 2 3 23 ABSTRACT: The morphology of the complete larval stage series of the crab Ergasticus 4 5 24 clouei is described and illustrated based on larvae (zoea I, zoea II and megalopa) 6 7 25 captured from plankton samples taken in Mediterranean waters. -
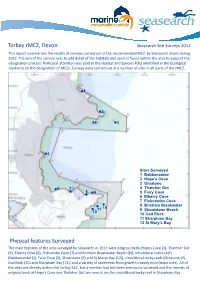
Torbay Rmcz, Devon Physical Features Surveyed
Torbay rMCZ, Devon Seasearch Site Surveys 2012 This report summarises the results of surveys carried out in the recommended MCZ by Seasearch divers during 2012. The aim of the surveys was to add detail of the habitats and species found within the area to support the designation process. Particular attention was paid to the Habitat and Species FOCI identified in the Ecological Guidance on the designation of MCZs. Surveys were carried out at a number of sites in all parts of the rMCZ. 1 2 4 3 5 Sites Surveyed 1 Babbacombe 2 Hope’s Cove CW 3 Orestone 6 7 4 Thatcher Gut 8 9 5 Fairy Cove 6 Elberry Cove 7 Fishcombe Cove 8 Brixham Breakwater 10 11 9 Shoalstone Beach 12 10 Cod Rock 11 Sharpham Bay 12 St Mary’s Bay Physical features Surveyed The main features of the area surveyed by Seasearch in 2012 were eelgrass beds (Hopes Cove (2), Thatcher Gut (4), Elberry Cove (6), Fishcombe Cove (7) and Brixham Breakwater Beach (8)), infralittoral rocky reefs (Babbacombe (1), Fairy Cove (5), Shoalstone (9) and St Marys Bay (12)), circalittoral rocky reefs (Orestone (3), Cod Rock (10) and Sharpham Bay (11)) and a variety of sediments from gravel to sandy mud (most sites). All of the sites are already within the Torbay SAC, but a number had not been previously surveyed and the records of eelgrass beds at Hope’s Cove and Thatcher Gut are new, is arCWe the circalittoral rocky reef in Sharpham Bay. CW Features of the Marine Life and Habitats Eeelgrass Beds (Sites 2,4,6,7,8) Eelgrass, Zostera marina, is one of the very few flowering plants underwater and, unlike seaweeds, has a system of roots which can help to bind soft sediments and create a complex habitat in what would otherwise be a relatively un-diverse environment. -

Decapoda, Brachyura
APLICACIÓN DE TÉCNICAS MORFOLÓGICAS Y MOLECULARES EN LA IDENTIFICACIÓN DE LA MEGALOPA de Decápodos Braquiuros de la Península Ibérica bérica I enínsula P raquiuros de la raquiuros B ecápodos D de APLICACIÓN DE TÉCNICAS MORFOLÓGICAS Y MOLECULARES EN LA IDENTIFICACIÓN DE LA MEGALOPA LA DE IDENTIFICACIÓN EN LA Y MOLECULARES MORFOLÓGICAS TÉCNICAS DE APLICACIÓN Herrero - MEGALOPA “big eyes” Leach 1793 Elena Marco Elena Marco-Herrero Programa de Doctorado en Biodiversidad y Biología Evolutiva Rd. 99/2011 Tesis Doctoral, Valencia 2015 Programa de Doctorado en Biodiversidad y Biología Evolutiva Rd. 99/2011 APLICACIÓN DE TÉCNICAS MORFOLÓGICAS Y MOLECULARES EN LA IDENTIFICACIÓN DE LA MEGALOPA DE DECÁPODOS BRAQUIUROS DE LA PENÍNSULA IBÉRICA TESIS DOCTORAL Elena Marco-Herrero Valencia, septiembre 2015 Directores José Antonio Cuesta Mariscal / Ferran Palero Pastor Tutor Álvaro Peña Cantero Als naninets AGRADECIMIENTOS-AGRAÏMENTS Colaboración y ayuda prestada por diferentes instituciones: - Ministerio de Ciencia e Innovación (actual Ministerio de Economía y Competitividad) por la concesión de una Beca de Formación de Personal Investigador FPI (BES-2010- 033297) en el marco del proyecto: Aplicación de técnicas morfológicas y moleculares en la identificación de estados larvarios planctónicos de decápodos braquiuros ibéricos (CGL2009-11225) - Departamento de Ecología y Gestión Costera del Instituto de Ciencias Marinas de Andalucía (ICMAN-CSIC) - Club Náutico del Puerto de Santa María - Centro Andaluz de Ciencias y Tecnologías Marinas (CACYTMAR) - Instituto Español de Oceanografía (IEO), Centros de Mallorca y Cádiz - Institut de Ciències del Mar (ICM-CSIC) de Barcelona - Institut de Recerca i Tecnología Agroalimentàries (IRTA) de Tarragona - Centre d’Estudis Avançats de Blanes (CEAB) de Girona - Universidad de Málaga - Natural History Museum of London - Stazione Zoologica Anton Dohrn di Napoli (SZN) - Universitat de Barcelona AGRAÏSC – AGRADEZCO En primer lugar quisiera agradecer a mis directores, el Dr. -

A History of the British Stalk-Eyed Crustacea
Go ygle Acerca de este libro Esta es una copia digital de un libro que, durante generaciones, se ha conservado en las estanterias de una biblioteca, hasta que Google ha decidido escanearlo como parte de un proyecto que pretende que sea posible descubrir en linea libros de todo el mundo. Ha sobrevivido tantos anos como para que los derechos de autor hayan expirado y el libro pase a ser de dominio publico. El que un libro sea de dominio publico significa que nunca ha estado protegido por derechos de autor, o bien que el periodo legal de estos derechos ya ha expirado. Es posible que una misma obra sea de dominio publico en unos paises y, sin embargo, no lo sea en otros. Los libros de dominio publico son nuestras puertas hacia el pasado, suponen un patrimonio historico, cultural y de conocimientos que, a menudo, resulta dificil de descubrir. Todas las anotaciones, marcas y otras senales en los margenes que esten presentes en el volumen original apareceran tambien en este archivo como testimonio del largo viaje que el libro ha recorrido desde el editor hasta la biblioteca y, finalmente, hasta usted. Normas de uso Google se enorgullece de poder colaborar con distintas bibliotecas para digitalizar los materiales de dominio publico a fin de hacerlos accesibles a todo el mundo. Los libros de dominio publico son patrimonio de todos, nosotros somos sus humildes guardianes. No obstante, se trata de un trabajo caro. Por este motivo, y para poder ofrecer este recurso, hemos tornado medidas para evitar que se produzca un abuso por parte de terceros con fines comerciales, y hemos incluido restricciones tecnicas sobre las solicitudes automatizadas. -

Larval Growth
LARVAL GROWTH Edited by ADRIAN M.WENNER University of California, Santa Barbara OFFPRINT A.A.BALKEMA/ROTTERDAM/BOSTON DARRYL L.FELDER* / JOEL W.MARTIN** / JOSEPH W.GOY* * Department of Biology, University of Louisiana, Lafayette, USA ** Department of Biological Science, Florida State University, Tallahassee, USA PATTERNS IN EARLY POSTLARVAL DEVELOPMENT OF DECAPODS ABSTRACT Early postlarval stages may differ from larval and adult phases of the life cycle in such characteristics as body size, morphology, molting frequency, growth rate, nutrient require ments, behavior, and habitat. Primarily by way of recent studies, information on these quaUties in early postlarvae has begun to accrue, information which has not been previously summarized. The change in form (metamorphosis) that occurs between larval and postlarval life is pronounced in some decapod groups but subtle in others. However, in almost all the Deca- poda, some ontogenetic changes in locomotion, feeding, and habitat coincide with meta morphosis and early postlarval growth. The postmetamorphic (first postlarval) stage, here in termed the decapodid, is often a particularly modified transitional stage; terms such as glaucothoe, puerulus, and megalopa have been applied to it. The postlarval stages that fol low the decapodid successively approach more closely the adult form. Morphogenesis of skeletal and other superficial features is particularly apparent at each molt, but histogenesis and organogenesis in early postlarvae is appreciable within intermolt periods. Except for the development of primary and secondary sexual organs, postmetamorphic change in internal anatomy is most pronounced in the first several postlarval instars, with the degree of anatomical reorganization and development decreasing in each of the later juvenile molts. -

Padrões De Distribuição Da Abundância Larvar De Crustáceos Decápodes Na Baía De Cascais
UNIVERSIDADE DO ALGARVE FACULDADE DE CIÊNCIAS DO MAR E DO AMBIENTE PADRÕES DE DISTRIBUIÇÃO DA ABUNDÂNCIA LARVAR DE CRUSTÁCEOS DECÁPODES NA BAÍA DE CASCAIS DISSERTAÇÃO PARA A OBTENÇÃO DO GRAU DE MESTRE EM BIOLOGIA MARINHA ESPECIALIZAÇÃO EM ECOLOGIA E CONSERVAÇÃO MARINHA CARLA ISABEL DE ALMEIDA SANTINHO FARO 2009 UNIVERSIDADE DO ALGARVE FACULDADE DE CIÊNCIAS DO MAR E DO AMBIENTE PADRÕES DE DISTRIBUIÇÃO DA ABUNDÂNCIA LARVAR DE CRUSTÁCEOS DECÁPODES NA BAÍA DE CASCAIS DISSERTAÇÃO PARA A OBTENÇÃO DO GRAU DE MESTRE EM BIOLOGIA MARINHA ESPECIALIZAÇÃO EM ECOLOGIA E CONSERVAÇÃO MARINHA CARLA ISABEL DE ALMEIDA SANTINHO FARO 2009 Dissertação realizada no Instituto Nacional dos Recursos Biológicos – IPIMAR (Lisboa) entre Abril de 2007 e Fevereiro de 2009, Orientada por: Doutora Antonina dos Santos Investigadora do INRB-IPIMAR e Doutora Margarida Castro Professora associada da Faculdade de Ciências do Mar e do Ambiente da Universidade do Algarve. Agradecimentos Gostaria de agradecer a todas as pessoas que tornaram possível a realização deste trabalho. Em primeiro lugar o meu agradecimento muito especial à minha orientadora Doutora Antonina dos Santos que tão bem me aconselhou durante a realização deste trabalho. Muito obrigado por ter acreditado sempre, pelo apoio e incentivo, pela paciência, por me ensinado tanto e por estar sempre disponível para me ajudar. O meu enorme obrigado à Professora Doutora Margarida Castro por ter aceite ser minha orientadora e por me ter amparado sempre perante as dificuldades que surgiram desde o início. Muito obrigado pela confiança, pela paciência, pela sua disponibilidade e pelos conhecimentos que me transmitiu. Agradeço às minhas colegas de gabinete e laboratório, Cátia e Joana, por me terem acolhido tão bem e me terem ajudado em tudo e mais alguma coisa sempre que precisei. -

ECOLOGIA POPULACIONAL DE Libinia Ferreirae (BRACHYURA: MAJOIDEA) NO LITORAL SUDESTE DO BRASIL
UNIVERSIDADE ESTADUAL PAULISTA INSTITUTO DE BIOCIÊNCIAS GESLAINE RAFAELA LEMOS GONÇALVES ECOLOGIA POPULACIONAL DE Libinia ferreirae (BRACHYURA: MAJOIDEA) NO LITORAL SUDESTE DO BRASIL DISSERTAÇÃO DE MESTRADO BOTUCATU 2016 UNIVERSIDADE ESTADUAL PAULISTA INSTITUTO DE BIOCIÊNCIAS DISSERTAÇÃO DE MESTRADO Ecologia populacional de Libinia ferreirae (Brachyura: Majoidea) no litoral sudeste do Brasil Geslaine Rafaela Lemos Gonçalves Orientador: Prof. Dr. Antonio Leão Castilho Coorientadora: Profª. Drª. Maria Lucia Negreiros Fransozo Dissertação apresentada ao Instituto de Biociências da Universidade Estadual Paulista “Júlio de Mesquita Filho” – UNESP – Câmpus Botucatu, como parte dos requisitos para obtenção do Título de Mestre em Ciências, curso de Pós-graduação em Ciências Biológicas, Área de Concentração: Zoologia. Botucatu – SP 2016 FICHA CATALOGRÁFICA ELABORADA PELA SEÇÃO TÉC. AQUIS. TRATAMENTO DA INFORM. DIVISÃO TÉCNICA DE BIBLIOTECA E DOCUMENTAÇÃO - CÂMPUS DE BOTUCATU - UNESP BIBLIOTECÁRIA RESPONSÁVEL: ROSEMEIRE APARECIDA VICENTE-CRB 8/5651 Gonçalves, Geslaine Rafaela Lemos. Ecologia populacional de Libinia ferreirae (Brachyura: Majoidea) no litoral sudeste do Brasil / Geslaine Rafaela Lemos Gonçalves. - Botucatu, 2016 Dissertação (mestrado) - Universidade Estadual Paulista "Júlio de Mesquita Filho", Instituto de Biociências de Botucatu Orientador: Antonio Leão Castilho Coorientador: Maria Lucia Negreiros Fransozo Capes: 20402007 1. Caranguejo. 2. Dinâmica populacional. 3. Hábitos alimentares. 4. Ecologia de populações. 5. Epizoísmo. Palavras-chave: Ciclo de vida; Crescimento; Dinâmica populacional; Epizoísmo; Hábitos alimentares. NEBECC Núcleo de Estudos em Biologia, Ecologia e Cultivo de Crustáceos II “Sou biólogo e viajo pela savana de meu país. Nessa região encontro gente que não sabe ler livros. Mas que sabe ler o mundo. Nesse universo de outros saberes, sou eu o analfabeto” Mia Couto “O saber a gente aprende com os mestres e com os livros. -

Chaceon Inglei.Indd
1 ISSN 0523 - 7904 B O C A G I A N A Museu Municipal do Funchal (História Natural) Madeira 31.XII.2009 No. 230 FIRST RECORD OF THE DEEP-SEA RED CRAB CHACEON INGLEI (DECAPODA: GERYONIDAE) FROM MADEIRA AND THE CANARY ISLANDS (NORTHEASTERN ATLANTIC OCEAN) * R. ARAÚJO 1, M. BISCOITO 1, J. I. SANTANA 2 & J. A. GONZÁLEZ 2 With 2 figures and 1 table ABSTRACT. The deep-sea red crab Chaceon inglei Manning & Holthuis, 1989 is recorded for the first time from the waters of Madeira and the Canary Islands. This is the second species of the genus Chaceon to occur in Madeira Island and the third in the Canaries. These collections set a new bathymetric record for this species and represent the southern limit of its distribution in the eastern Atlantic Ocean. KEY WORDS: Crustacea, Decapoda, Geryonidae, Chaceon inglei, new record, Madeira, Canary Islands, NE Atlantic Ocean. 1 Museu Municipal do Funchal (História Natural), Rua da Mouraria, 31, 9000-047 Funchal, Madeira, Portugal. E-mail: [email protected] 2 Grupo de Biología Pesquera, Instituto Canario de Ciencias Marinas (ICCM – ACIISI), P. O. Box 56, Telde, 35200 Las Palmas, Canary Islands, Spain. * Contribution no. 150 of the Marine Biology Station of Funchal. 2 RESUMO. Neste artigo é assinalada pela primeira vez para as ilhas da Madeira e das Canárias uma nova espécie de caranguejo da fundura, Chaceon inglei Manning & Holthuis, 1989. Esta é a segunda espécie do género Chaceon assinalada para a ilha da Madeira e a terceira para as ilhas Canárias. Estas colheitas estabelecem um novo limite Sul de distribuição no Oceano Atlântico nordeste e constituem as mais profundas desta espécie registadas até ao presente. -

Post-Larval Developm Ent and Sexual Dim Orphism of the Spider Crab M
SciENTiA M arina 73(4) December 2009, 797-808, Barcelona (Spain) ISSN: 0214-8358 doi: 10.3989/scimar.2009.73n4797 Post-larval development and sexual dimorphism of the spider crab Maja brachydactyla (Brachyura: Majidae) GUILLERMO GUERAO and GUIOMAR ROTLLANT IRTA, Unitat de Cultius Experimentáis. Ctra. Pohle Nou, km 5.5, 43540 Sant Caries de la Rápita, Tarragona, Spain. E-mail: [email protected] SUMMARY: The post-larval development of the majid crab Maja brachydactyla Balss, 1922 was studied using laboratory- reared larvae obtained from adult individuals collected in the NE Atlantic. The morphology of the first juvenile stage is described in detail, while the most relevant morphological changes and sexual differentiation are highlighted for subsequent juvenile stages, until juvenile 8. The characteristic carapace spines of the adult phase are present in the first juvenile stage, though with great differences in the degree of development and relative size. The carapace shows a high length/weight ratio, which becomes similar to that of adults at stage 7-8. Males and females can be distinguished from juvenile stage 4. based on sexual dimorphism in the pleopods and the presence of gonopores. In addition, the allometric growth of the pleon is sex-dependent from juvenile stage 4. with females showing a positive allometry (b=1.23) and males an isometric allometry (b=1.02). Keywords'. Majidae. Maja brachydactyla, morphology, juvenile, post-larval development, growth, sexual dimorphism. RESUMEN: D esarrollo postlarvario y dimorfismo sexual de M a j a brachydactyia (Brachyura: M ajidae). - El desarrollo postlarvario del májido Maja brachydactyla ha sido estudiado en el laboratorio después del cultivo larvario realizado a partir de individuos adultos capturados en el NE del Atlántico. -

The Marine Arthropods of Turkey
Turkish Journal of Zoology Turk J Zool (2014) 38: http://journals.tubitak.gov.tr/zoology/ © TÜBİTAK Research Article doi:10.3906/zoo-1405-48 The marine arthropods of Turkey 1, 1 1 2 Ahmet Kerem BAKIR *, Tuncer KATAĞAN , Halim Vedat AKER , Tahir ÖZCAN , 3 4 1 1 Murat SEZGİN , Abdullah Suat ATEŞ , Cengiz KOÇAK , Fevzi KIRKIM 1 Faculty of Fisheries, Ege University, İzmir, Turkey 2 Faculty of Marine Sciences and Technology, Mustafa Kemal University, İskenderun, Hatay, Turkey 3 Faculty of Fisheries, Sinop University, Sinop, Turkey 4 Faculty of Marine Sciences and Technology, Çanakkale Onsekiz Mart University, Çanakkale, Turkey Received: 29.05.2014 Accepted: 30.07.2014 Published Online: 00.00.2013 Printed: 00.00.2013 Abstract: This recent checklist of marine arthropods found on the coasts of Turkey represents a total of 1531 species belonging to 7 classes: Malacostraca (766 species), Maxillopoda (437 species), Ostracoda (263 species), Pycnogonida (27 species), Arachnida (26 species), Branchiopoda (7 species), and Insecta (5 species). Seventy-five species were classified as alien species in the region. This paper also includes the first record of the amphipod Melita valesi from the Levantine coast of Turkey (Kaş, Gulf of Antalya). Key words: Arthropoda, Black Sea, Sea of Marmara, Aegean Sea, Levantine Sea, Turkey 1. Introduction İzmir Bay (Smirnæ) and the Bosphorus (Constantinopoli). The arthropods, containing approximately 1.2 million Forskål died of malaria in July 1763 and Carsten Niebuhr described species and constituting almost 80% of all edited and published the work of his friend in 1775. In described living animal species, constitute the largest the 19th century, Ostroumoff (1896) participated in the and most successful of the animal phyla.