The Phenetic Affinities of Rungwecebus Kipunji
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Targets, Tactics, and Cooperation in the Play Fighting of Two Genera of Old World Monkeys (Mandrillus and Papio): Accounting for Similarities and Differences
2019, 32 Heather M. Hill Editor Peer-reviewed Targets, Tactics, and Cooperation in the Play Fighting of Two Genera of Old World Monkeys (Mandrillus and Papio): Accounting for Similarities and Differences Kelly L. Kraus1, Vivien C. Pellis1, and Sergio M. Pellis1 1 Department of Neuroscience, University of Lethbridge, Canada Play fighting in many species involves partners competing to bite one another while avoiding being bitten. Species can differ in the body targets that are bitten and the tactics used to attack and defend those targets. However, even closely related species that attack and defend the same body target using the same tactics can differ markedly in how much the competitiveness of such interactions is mitigated by cooperation. A degree of cooperation is necessary to ensure that some turn-taking between the roles of attacker and defender occurs, as this is critical in preventing play fighting from escalating into serious fighting. In the present study, the dyadic play fighting of captive troops of 4 closely related species of Old World monkeys, 2 each from 2 genera of Papio and Mandrillus, was analyzed. All 4 species have a comparable social organization, are large bodied with considerable sexual dimorphism, and are mostly terrestrial. In all species, the target of biting is the same – the area encompassing the upper arm, shoulder, and side of the neck – and they have the same tactics of attack and defense. However, the Papio species exhibit more cooperation in their play than do the Mandrillus species, with the former using tactics that make biting easier to attain and that facilitate close bodily contact. -

Body Measurements for the Monkeys of Bioko Island, Equatorial Guinea
Primate Conservation 2009 (24): 99–105 Body Measurements for the Monkeys of Bioko Island, Equatorial Guinea Thomas M. Butynski¹,², Yvonne A. de Jong² and Gail W. Hearn¹ ¹Bioko Biodiversity Protection Program, Drexel University, Philadelphia, PA, USA ²Eastern Africa Primate Diversity and Conservation Program, Nanyuki, Kenya Abstract: Bioko Island, Equatorial Guinea, has a rich (eight genera, 11 species), unique (seven endemic subspecies), and threat- ened (five species) primate fauna, but the taxonomic status of most forms is not clear. This uncertainty is a serious problem for the setting of priorities for the conservation of Bioko’s (and the region’s) primates. Some of the questions related to the taxonomic status of Bioko’s primates can be resolved through the statistical comparison of data on their body measurements with those of their counterparts on the African mainland. Data for such comparisons are, however, lacking. This note presents the first large set of body measurement data for each of the seven species of monkeys endemic to Bioko; means, ranges, standard deviations and sample sizes for seven body measurements. These 49 data sets derive from 544 fresh adult specimens (235 adult males and 309 adult females) collected by shotgun hunters for sale in the bushmeat market in Malabo. Key Words: Bioko Island, body measurements, conservation, monkeys, morphology, taxonomy Introduction gordonorum), and surprisingly few such data exist even for some of the more widespread species (for example, Allen’s Comparing external body measurements for adult indi- swamp monkey Allenopithecus nigroviridis, northern tala- viduals from different sites has long been used as a tool for poin monkey Miopithecus ogouensis, and grivet Chlorocebus describing populations, subspecies, and species of animals aethiops). -

The Taxonomy of Primates in the Laboratory Context
P0800261_01 7/14/05 8:00 AM Page 3 C HAPTER 1 The Taxonomy of Primates T HE T in the Laboratory Context AXONOMY OF P Colin Groves RIMATES School of Archaeology and Anthropology, Australian National University, Canberra, ACT 0200, Australia 3 What are species? D Taxonomy: EFINITION OF THE The biological Organizing nature species concept Taxonomy means classifying organisms. It is nowadays commonly used as a synonym for systematics, though Disagreement as to what precisely constitutes a species P strictly speaking systematics is a much broader sphere is to be expected, given that the concept serves so many RIMATE of interest – interrelationships, and biodiversity. At the functions (Vane-Wright, 1992). We may be interested basis of taxonomy lies that much-debated concept, the in classification as such, or in the evolutionary implica- species. tions of species; in the theory of species, or in simply M ODEL Because there is so much misunderstanding about how to recognize them; or in their reproductive, phys- what a species is, it is necessary to give some space to iological, or husbandry status. discussion of the concept. The importance of what we Most non-specialists probably have some vague mean by the word “species” goes way beyond taxonomy idea that species are defined by not interbreeding with as such: it affects such diverse fields as genetics, biogeog- each other; usually, that hybrids between different species raphy, population biology, ecology, ethology, and bio- are sterile, or that they are incapable of hybridizing at diversity; in an era in which threats to the natural all. Such an impression ultimately derives from the def- world and its biodiversity are accelerating, it affects inition by Mayr (1940), whereby species are “groups of conservation strategies (Rojas, 1992). -

Controlled Animals
Environment and Sustainable Resource Development Fish and Wildlife Policy Division Controlled Animals Wildlife Regulation, Schedule 5, Part 1-4: Controlled Animals Subject to the Wildlife Act, a person must not be in possession of a wildlife or controlled animal unless authorized by a permit to do so, the animal was lawfully acquired, was lawfully exported from a jurisdiction outside of Alberta and was lawfully imported into Alberta. NOTES: 1 Animals listed in this Schedule, as a general rule, are described in the left hand column by reference to common or descriptive names and in the right hand column by reference to scientific names. But, in the event of any conflict as to the kind of animals that are listed, a scientific name in the right hand column prevails over the corresponding common or descriptive name in the left hand column. 2 Also included in this Schedule is any animal that is the hybrid offspring resulting from the crossing, whether before or after the commencement of this Schedule, of 2 animals at least one of which is or was an animal of a kind that is a controlled animal by virtue of this Schedule. 3 This Schedule excludes all wildlife animals, and therefore if a wildlife animal would, but for this Note, be included in this Schedule, it is hereby excluded from being a controlled animal. Part 1 Mammals (Class Mammalia) 1. AMERICAN OPOSSUMS (Family Didelphidae) Virginia Opossum Didelphis virginiana 2. SHREWS (Family Soricidae) Long-tailed Shrews Genus Sorex Arboreal Brown-toothed Shrew Episoriculus macrurus North American Least Shrew Cryptotis parva Old World Water Shrews Genus Neomys Ussuri White-toothed Shrew Crocidura lasiura Greater White-toothed Shrew Crocidura russula Siberian Shrew Crocidura sibirica Piebald Shrew Diplomesodon pulchellum 3. -
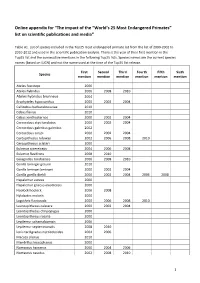
Online Appendix for “The Impact of the “World's 25 Most Endangered
Online appendix for “The impact of the “World’s 25 Most Endangered Primates” list on scientific publications and media” Table A1. List of species included in the Top25 most endangered primate list from the list of 2000-2002 to 2010-2012 and used in the scientific publication analysis. There is the year of their first mention in the Top25 list and the consecutive mentions in the following Top25 lists. Species names are the current species names (based on IUCN) and not the name used at the time of the Top25 list release. First Second Third Fourth Fifth Sixth Species mention mention mention mention mention mention Ateles fusciceps 2006 Ateles hybridus 2006 2008 2010 Ateles hybridus brunneus 2004 Brachyteles hypoxanthus 2000 2002 2004 Callicebus barbarabrownae 2010 Cebus flavius 2010 Cebus xanthosternos 2000 2002 2004 Cercocebus atys lunulatus 2000 2002 2004 Cercocebus galeritus galeritus 2002 Cercocebus sanjei 2000 2002 2004 Cercopithecus roloway 2002 2006 2008 2010 Cercopithecus sclateri 2000 Eulemur cinereiceps 2004 2006 2008 Eulemur flavifrons 2008 2010 Galagoides rondoensis 2006 2008 2010 Gorilla beringei graueri 2010 Gorilla beringei beringei 2000 2002 2004 Gorilla gorilla diehli 2000 2002 2004 2006 2008 Hapalemur aureus 2000 Hapalemur griseus alaotrensis 2000 Hoolock hoolock 2006 2008 Hylobates moloch 2000 Lagothrix flavicauda 2000 2006 2008 2010 Leontopithecus caissara 2000 2002 2004 Leontopithecus chrysopygus 2000 Leontopithecus rosalia 2000 Lepilemur sahamalazensis 2006 Lepilemur septentrionalis 2008 2010 Loris tardigradus nycticeboides -

Patterns of Cranial Shape Variation in the Papionini (Primates
Michelle Singleton Patterns of cranial shape variation in the Department of Anatomy, Papionini (Primates: Cercopithecinae) Midwestern University, 555 31st Street, Downers Grove, Traditional classifications of the Old World monkey tribe Papionini Illinois 60515, U.S.A. (Primates: Cercopithecinae) recognized the mangabey genera E-mail: Cercocebus and Lophocebus as sister taxa. However, molecular studies [email protected] have consistently found the mangabeys to be diphyletic, with Cercocebus and Mandrillus forming a clade to the exclusion of all other Received 10 January 2001 papionins. Recent studies have identified cranial and postcranial Revision received features which distinguish the Cercocebus–Mandrillus clade, however 26 October 2001 and the detailed similarities in cranial shape between the mangabey accepted 12 December 2001 genera are more difficult to reconcile with the molecular evidence. ff Keywords: Papionini, Given the large size di erential between members of the papionin molecular clades, it has frequently been suggested that allometric mangabey diphyly, cranial ff homoplasy, allometry, e ects account for homoplasy in papionin cranial form. A combina- geometric morphometrics. tion of geometric morphometric, bivariate, and multivariate methods was used to evaluate the hypothesis that allometric scaling contributes to craniofacial similarities between like-sized papionin taxa. Patterns of allometric and size-independent cranial shape variation were subsequently described and related to known papionin phylogenetic relationships and patterns of development. Results confirm that allometric scaling of craniofacial shape characterized by positive facial allometry and negative neurocranial allometry is present across adult papionins. Pairwise comparisons of regression lines among genera revealed considerable homogeneity of scaling within the Papionini, however statistically significant differ- ences in regression lines also were noted. -

Mandrillus Leucophaeus Poensis)
Ecology and Behavior of the Bioko Island Drill (Mandrillus leucophaeus poensis) A Thesis Submitted to the Faculty of Drexel University by Jacob Robert Owens in partial fulfillment of the requirements for the degree of Doctor of Philosophy December 2013 i © Copyright 2013 Jacob Robert Owens. All Rights Reserved ii Dedications To my wife, Jen. iii Acknowledgments The research presented herein was made possible by the financial support provided by Primate Conservation Inc., ExxonMobil Foundation, Mobil Equatorial Guinea, Inc., Margo Marsh Biodiversity Fund, and the Los Angeles Zoo. I would also like to express my gratitude to Dr. Teck-Kah Lim and the Drexel University Office of Graduate Studies for the Dissertation Fellowship and the invaluable time it provided me during the writing process. I thank the Government of Equatorial Guinea, the Ministry of Fisheries and the Environment, Ministry of Information, Press, and Radio, and the Ministry of Culture and Tourism for the opportunity to work and live in one of the most beautiful and unique places in the world. I am grateful to the faculty and staff of the National University of Equatorial Guinea who helped me navigate the geographic and bureaucratic landscape of Bioko Island. I would especially like to thank Jose Manuel Esara Echube, Claudio Posa Bohome, Maximilliano Fero Meñe, Eusebio Ondo Nguema, and Mariano Obama Bibang. The journey to my Ph.D. has been considerably more taxing than I expected, and I would not have been able to complete it without the assistance of an expansive list of people. I would like to thank all of you who have helped me through this process, many of whom I lack the space to do so specifically here. -

The Reproductive Trajectories of Bachelor Geladas
THE REPRODUCTIVE TRAJECTORIES OF BACHELOR GELADAS by David J. Pappano A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Anthropology) in The University of Michigan 2013 Doctoral Committee: Professor Jacinta C. Beehner, Chair Professor Abigail W. Bigham Professor John C. Mitani Professor Elizabeth A. Tibbetts © David J. Pappano All rights reserved 2013 DEDICATION For my parents, Teresa and Donald Pappano For indulging a child’s curiosity in bats, snakes, and other animals described in Golden Guides to Nature. Most parents would have just read fairy tales to their children. And my best friend and partner, Ashley For your patience, kindness, and love in years past and those yet to come. ii ACKNOWLEDGEMENTS Over the past six years, I have been fortunate to have the support of friends, family, colleagues, and funding agencies that have made this dissertation possible. I am grateful for their input at all levels of this endeavor—from conception to completion. My time at the University of Michigan has been among the most formative and memorable experiences of my life, and I hope that many of the friends and colleagues I have met over these past years will continue to be a part of my life in the years to come. This research would not have been possible without the generous support of several government and non-profit funding agencies. A Graduate Research Fellowship from the National Science Foundation and the James Spuhler Fellowship through the Department of Anthropology largely funded my studies at Michigan. I am also grateful to the National Science Foundation for support through a Doctoral Dissertation Improvement Grant. -

Hormonal and Morphological Aspects of Growth and Sexual Maturation in Wild-Caught Male Vervet Monkeys (Chlorocebus Aethiops Pygerythrus) Tegan J
University of Wisconsin Milwaukee UWM Digital Commons Theses and Dissertations December 2012 Hormonal and Morphological Aspects of Growth and Sexual Maturation in Wild-Caught Male Vervet Monkeys (Chlorocebus aethiops pygerythrus) Tegan J. Gaetano University of Wisconsin-Milwaukee Follow this and additional works at: https://dc.uwm.edu/etd Part of the Animal Sciences Commons, Developmental Biology Commons, and the Endocrinology Commons Recommended Citation Gaetano, Tegan J., "Hormonal and Morphological Aspects of Growth and Sexual Maturation in Wild-Caught Male Vervet Monkeys (Chlorocebus aethiops pygerythrus)" (2012). Theses and Dissertations. 30. https://dc.uwm.edu/etd/30 This Thesis is brought to you for free and open access by UWM Digital Commons. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of UWM Digital Commons. For more information, please contact [email protected]. HORMONAL AND MORPHOLOGICAL ASPECTS OF GROWTH AND SEXUAL MATURATION IN WILD-CAUGHT MALE VERVET MONKEYS (CHLOROCEBUS AETHIOPS PYGERYTHRUS) by Tegan J. Gaetano A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in Anthropology at The University of Wisconsin at Milwaukee December 2012 ABSTRACT ENDOCRINE AND MORPHOLOGICAL ASPECTS OF GROWTH AND SEXUAL MATURATION IN WILD-CAUGHT MALE VERVET MONKEYS (CHLOROCEBUS AETHIOPS PYGERYTHRUS) by Tegan J. Gaetano The University of Wisconsin at Milwaukee, 2012 Under the Supervision of Dr. Trudy R. Turner Knowledge of baseline changes in testosterone (T) and other androgens is central to both investigations of morphological, physiological, and behavioral correlates of inter-individual variation in the timing and shape of key events and transitions over the life course and questions of the evolution of species-specific schedules of maturation in primates. -

View / Open Gartland Oregon 0171A 12939.Pdf
MALE JAPANESE MACAQUE (MACACA FUSCATA) SOCIALITY: BEHAVIORAL STRATEGIES AND WELFARE SCIENCE APPLICATIONS by KYLEN NADINE GARTLAND A DISSERTATION Presented to the Department of Anthropology and the Graduate School of the University of Oregon in partial fulfillment of the requirements for the degree of Doctor of Philosophy March 2021 DISSERTATION APPROVAL PAGE Student: Kylen Nadine Gartland Title: Male Japanese Macaque (Macaca fuscata) Sociality: Behavioral Strategies and Welfare Science Applications This dissertation has been accepted and approved in partial fulfillment of the requirements for the Doctor of Philosophy degree in the Department of Anthropology by: Frances White Chairperson Lawrence Ulibarri Core Member Steve Frost Core Member Renee Irvin Institutional Representative and Kate Mondlock Interim Vice Provost and Dean of the Graduate School Original approval signatures are on file with the University of Oregon Graduate School. Degree awarded March 2021 ii © 2021 Kylen Nadine Gartland iii DISSERTATION ABSTRACT Kylen Nadine Gartland Doctor of Philosophy Department of Anthropology February 2021 Title: Male Japanese macaque (Macaca fuscata) sociality: Behavioral strategies and welfare science application Evolutionarily, individuals should pursue social strategies which confer advantages such as coalitionary support, mating opportunities, or access to limited resources. How an individual forms and maintains social bonds may be influenced by a large number of factors including sex, age, dominance rank, group structure, group demographics, relatedness, or seasonality. Individuals may employ differential social strategies both in terms of the type and quantity of interactions they engage in as well as their chosen social partners. The objective of this dissertation is to examine sociality in adult male Japanese macaques (Macaca fuscata) and the varying strategies that individuals may employ depending on their relative position within a social group. -

Population Genomics of a Baboon Hybrid Zone in Zambia Kenneth Lyu Chiou Washington University in St
Washington University in St. Louis Washington University Open Scholarship Arts & Sciences Electronic Theses and Dissertations Arts & Sciences Spring 5-15-2017 Population Genomics of a Baboon Hybrid Zone in Zambia Kenneth Lyu Chiou Washington University in St. Louis Follow this and additional works at: https://openscholarship.wustl.edu/art_sci_etds Part of the Biological and Physical Anthropology Commons, and the Genetics Commons Recommended Citation Chiou, Kenneth Lyu, "Population Genomics of a Baboon Hybrid Zone in Zambia" (2017). Arts & Sciences Electronic Theses and Dissertations. 1094. https://openscholarship.wustl.edu/art_sci_etds/1094 This Dissertation is brought to you for free and open access by the Arts & Sciences at Washington University Open Scholarship. It has been accepted for inclusion in Arts & Sciences Electronic Theses and Dissertations by an authorized administrator of Washington University Open Scholarship. For more information, please contact [email protected]. WASHINGTON UNIVERSITY IN ST. LOUIS Department of Anthropology Dissertation Examination Committee: Jane Phillips-Conroy, Chair Amy Bauernfeind Clifford Jolly Allan Larson Amanda Melin Population Genomics of a Baboon Hybrid Zone in Zambia By Kenneth L. Chiou A dissertation presented to The Graduate School of Washington University in partial fulfillment of the requirements for the degree of Doctor of Philosophy May 2017 St. Louis, Missouri © Kenneth L. Chiou Contents List of Figures v List of Tables vii Acknowledgments ix Abstract xiv 1 Hybrid Zones and Papio: A Review 1 Introduction . 1 Hybridization . 2 Study animals . 5 Evolution of genus Papio ................................. 7 Hybridization in Papio ................................... 15 Zambian Papio: diversity and distribution . 23 Relevance to Homo ..................................... 35 Study site . 40 Research summary . 44 Overview of upcoming chapters . -

Phylogenetic Relationships of Living and Fossil African Papionins: Combined Evidence from Morphology and Molecules
City University of New York (CUNY) CUNY Academic Works Publications and Research Hunter College 2018 Phylogenetic relationships of living and fossil African papionins: Combined evidence from morphology and molecules Kelsey D. Pugh The Graduate Center, City University of New York Christopher C. Gilbert CUNY Hunter College How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/hc_pubs/647 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] Journal of Human Evolution 123 (2018) 35e51 Contents lists available at ScienceDirect Journal of Human Evolution journal homepage: www.elsevier.com/locate/jhevol Phylogenetic relationships of living and fossil African papionins: Combined evidence from morphology and molecules * Kelsey D. Pugh a, b, , Christopher C. Gilbert a, b, c a PhD Program in Anthropology, Graduate Center of the City University of New York, 365 Fifth Avenue, New York, NY 10016, USA b New York Consortium in Evolutionary Primatology (NYCEP), USA c Department of Anthropology, Hunter College of the City University of New York, 695 Park Avenue, New York, NY 10065, USA article info abstract Article history: African papionins are a highly successful subtribe of Old World monkeys with an extensive fossil record. Received 7 November 2017 On the basis of both molecular and morphological data, crown African papionins are divided into two Accepted 1 June 2018 clades: Cercocebus/Mandrillus and Papio/Lophocebus/Rungwecebus/Theropithecus (P/L/R/T), though Available online 26 July 2018 phylogenetic relationships in the latter clade, among both fossil and extant taxa, remain difficult to resolve.