Mandrillus Leucophaeus Poensis)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

First Bolivian Record of Laughing Gull Leucophaeus Atricilla, and Two Noteworthy Records of Fulica Coots from Laguna Guapilo, Dpto
Cotinga 41 First Bolivian record of Laughing Gull Leucophaeus atricilla, and two noteworthy records of Fulica coots from Laguna Guapilo, dpto. Santa Cruz Matthew L. Brady, Anna E. Hiller, Damián I. Rumiz, Nanuq L. Herzog-Hamel and Sebastian K. Herzog Received 30 November 2018; fnal revision accepted 29 April 2019 Cotinga 41 (2019): 98–100 published online 21 June 2019 El 28 de enero de 2018, durante una visita a laguna Guapilo, al este de Santa Cruz de la Sierra, depto. Santa Cruz, Bolivia, observamos una Gaviota Reidora Leucophaeus atricilla, el primer registro en Bolivia. Adicionalmente, observamos comportamiento indicativo de anidación de la Gallareta Chica Fulica leucoptera, una especie que se consideraba como visitante no reproductiva en Bolivia, así como una Gallareta Andina Fulica ardesiaca, el primer registro para el depto. Santa Cruz. La reproducción de F. leucoptera en la laguna Guapilo fue confrmada el 5 de mayo de 2018 mediante la fotografía de un polluelo. On 28 January 2018, MLB, AEH, NLH-H and We aged the bird during the observation SKH observed several notable birds at Laguna based on the following combination of characters: Guapilo (17°46’50”S 63°05’48”W), a semi-urban uniformly dark primaries, without the white apical park 8.9 km east of Santa Cruz city centre, dpto. spots typical of older birds; a dark tail-band; Santa Cruz, Bolivia. The habitat is dominated by a extensive ash-grey neck and breast; and worn, c.35-ha lagoon, with dense mats of reeds and water brownish wing-coverts. These features are typical hyacinth Eichhornia crassipes at the edges, and of an advanced frst-year L. -

Body Measurements for the Monkeys of Bioko Island, Equatorial Guinea
Primate Conservation 2009 (24): 99–105 Body Measurements for the Monkeys of Bioko Island, Equatorial Guinea Thomas M. Butynski¹,², Yvonne A. de Jong² and Gail W. Hearn¹ ¹Bioko Biodiversity Protection Program, Drexel University, Philadelphia, PA, USA ²Eastern Africa Primate Diversity and Conservation Program, Nanyuki, Kenya Abstract: Bioko Island, Equatorial Guinea, has a rich (eight genera, 11 species), unique (seven endemic subspecies), and threat- ened (five species) primate fauna, but the taxonomic status of most forms is not clear. This uncertainty is a serious problem for the setting of priorities for the conservation of Bioko’s (and the region’s) primates. Some of the questions related to the taxonomic status of Bioko’s primates can be resolved through the statistical comparison of data on their body measurements with those of their counterparts on the African mainland. Data for such comparisons are, however, lacking. This note presents the first large set of body measurement data for each of the seven species of monkeys endemic to Bioko; means, ranges, standard deviations and sample sizes for seven body measurements. These 49 data sets derive from 544 fresh adult specimens (235 adult males and 309 adult females) collected by shotgun hunters for sale in the bushmeat market in Malabo. Key Words: Bioko Island, body measurements, conservation, monkeys, morphology, taxonomy Introduction gordonorum), and surprisingly few such data exist even for some of the more widespread species (for example, Allen’s Comparing external body measurements for adult indi- swamp monkey Allenopithecus nigroviridis, northern tala- viduals from different sites has long been used as a tool for poin monkey Miopithecus ogouensis, and grivet Chlorocebus describing populations, subspecies, and species of animals aethiops). -

The Taxonomy of Primates in the Laboratory Context
P0800261_01 7/14/05 8:00 AM Page 3 C HAPTER 1 The Taxonomy of Primates T HE T in the Laboratory Context AXONOMY OF P Colin Groves RIMATES School of Archaeology and Anthropology, Australian National University, Canberra, ACT 0200, Australia 3 What are species? D Taxonomy: EFINITION OF THE The biological Organizing nature species concept Taxonomy means classifying organisms. It is nowadays commonly used as a synonym for systematics, though Disagreement as to what precisely constitutes a species P strictly speaking systematics is a much broader sphere is to be expected, given that the concept serves so many RIMATE of interest – interrelationships, and biodiversity. At the functions (Vane-Wright, 1992). We may be interested basis of taxonomy lies that much-debated concept, the in classification as such, or in the evolutionary implica- species. tions of species; in the theory of species, or in simply M ODEL Because there is so much misunderstanding about how to recognize them; or in their reproductive, phys- what a species is, it is necessary to give some space to iological, or husbandry status. discussion of the concept. The importance of what we Most non-specialists probably have some vague mean by the word “species” goes way beyond taxonomy idea that species are defined by not interbreeding with as such: it affects such diverse fields as genetics, biogeog- each other; usually, that hybrids between different species raphy, population biology, ecology, ethology, and bio- are sterile, or that they are incapable of hybridizing at diversity; in an era in which threats to the natural all. Such an impression ultimately derives from the def- world and its biodiversity are accelerating, it affects inition by Mayr (1940), whereby species are “groups of conservation strategies (Rojas, 1992). -

This Draft Holds Additions and Corrections Made After 15
Thomas M. Butynski Lists of Publications, Reports and Consultancies Published papers (many available as pdfs, some at www.wildsolutions.nl) Butynski, T. M. 1973. Life history and economic value of the springhare (Pedetes capensis Forester) in Botswana. Botswana Notes and Records 5: 209–213. von Richter, W. & Butynski, T. M. 1973. Hunting in Botswana. Botswana Notes and Records 5: 191–208. Butynski, T. M. 1974. An encounter between African wild dog and domestic dog. African Journal of Ecology 12: 243. Butynski, T. M. 1974. In Botswana most of the meat is wild. Unasylva 26: 24–29. Butynski, T. M. 1975. Ageing black-backed jackal pups in Botswana. Journal of the Southern African Wildlife Management Association 5: 39–42. Butynski, T. M. & von Richter, W. 19975. Wildlife management in Botswana. Wildlife Society Bulletin 3: 19–24. Dawson, J. L. & Butynski, T. M. 1975. Khutse Game Reserve, Botswana: preserving the Kalahari ecosystem. Biological Conservation 7: 147–153. Butynski, T. M. 1979. Body and organ growth of the springhare Acta Theriologica 24: 431–448. Butynski, T. M. 1979. Reproductive ecology of the springhaas Pedetes capensis in Botswana. Journal of Zoology, London 189: 221–232. Butynski, T. M. & Hanks, J. 1979. Reproductive activity in the male springhare Pedetes capensis in Botswana. South African Journal of Wildlife Research 9: 13–17. Butynski, T. M. & Mattingly, R. 1979. Burrow structure and fossorial ecology of the springhare Pedetes capensis in Botswana. African Journal of Ecology 17: 205– 215. Butynski, T. M. 1980. Growth and development of the foetal springhare Pedetes capensis in Botswana. Mammalia 44: 361–369. -
![Arxiv:2101.10390V1 [Cs.LG] 25 Jan 2021 Terms of Quality, Increasing the Comparability and Reproducibil- Conservation and Education Initiatives](https://docslib.b-cdn.net/cover/0861/arxiv-2101-10390v1-cs-lg-25-jan-2021-terms-of-quality-increasing-the-comparability-and-reproducibil-conservation-and-education-initiatives-180861.webp)
Arxiv:2101.10390V1 [Cs.LG] 25 Jan 2021 Terms of Quality, Increasing the Comparability and Reproducibil- Conservation and Education Initiatives
Introducing a Central African Primate Vocalisation Dataset for Automated Species Classification Joeri A. Zwerts1, Jelle Treep2, Casper S. Kaandorp2, Floor Meewis1, Amparo C. Koot1, Heysem Kaya3 1 Department of Biology, Utrecht University, Utrecht, The Netherlands 2 Information and Technology Services, Utrecht University, Utrecht, The Netherlands 3 Department of Information and Computing Sciences, Utrecht University, Utrecht, The Netherlands [email protected], [email protected] Abstract classifier capable of detecting species in the wild. This may also provide insights into whether this approach, of using sanctuary Automated classification of animal vocalisations is a poten- recordings, can be used to train classifiers for other species as tially powerful wildlife monitoring tool. Training robust clas- well, to aid in the development of cost-effective monitoring to sifiers requires sizable annotated datasets, which are not eas- meet modern conservation challenges. ily recorded in the wild. To circumvent this problem, we In this paper, we present the dataset, the semi-automatic an- recorded four primate species under semi-natural conditions in notation process that we used to speed up the manual annotation a wildlife sanctuary in Cameroon with the objective to train a process, and a benchmark species classification system. classifier capable of detecting species in the wild. Here, we introduce the collected dataset, describe our approach and ini- tial results of classifier development. To increase the efficiency 1.1. Related Work of the annotation process, we condensed the recordings with Multiple studies have applied automatic acoustic monitoring for an energy/change based automatic vocalisation detection. Seg- a variety of taxa including cetaceans [4], birds [5], bats [6], menting the annotated chunks into training, validation and test insects [7], amphibians [8], and forest elephants [9]. -

Controlled Animals
Environment and Sustainable Resource Development Fish and Wildlife Policy Division Controlled Animals Wildlife Regulation, Schedule 5, Part 1-4: Controlled Animals Subject to the Wildlife Act, a person must not be in possession of a wildlife or controlled animal unless authorized by a permit to do so, the animal was lawfully acquired, was lawfully exported from a jurisdiction outside of Alberta and was lawfully imported into Alberta. NOTES: 1 Animals listed in this Schedule, as a general rule, are described in the left hand column by reference to common or descriptive names and in the right hand column by reference to scientific names. But, in the event of any conflict as to the kind of animals that are listed, a scientific name in the right hand column prevails over the corresponding common or descriptive name in the left hand column. 2 Also included in this Schedule is any animal that is the hybrid offspring resulting from the crossing, whether before or after the commencement of this Schedule, of 2 animals at least one of which is or was an animal of a kind that is a controlled animal by virtue of this Schedule. 3 This Schedule excludes all wildlife animals, and therefore if a wildlife animal would, but for this Note, be included in this Schedule, it is hereby excluded from being a controlled animal. Part 1 Mammals (Class Mammalia) 1. AMERICAN OPOSSUMS (Family Didelphidae) Virginia Opossum Didelphis virginiana 2. SHREWS (Family Soricidae) Long-tailed Shrews Genus Sorex Arboreal Brown-toothed Shrew Episoriculus macrurus North American Least Shrew Cryptotis parva Old World Water Shrews Genus Neomys Ussuri White-toothed Shrew Crocidura lasiura Greater White-toothed Shrew Crocidura russula Siberian Shrew Crocidura sibirica Piebald Shrew Diplomesodon pulchellum 3. -
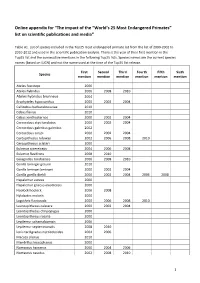
Online Appendix for “The Impact of the “World's 25 Most Endangered
Online appendix for “The impact of the “World’s 25 Most Endangered Primates” list on scientific publications and media” Table A1. List of species included in the Top25 most endangered primate list from the list of 2000-2002 to 2010-2012 and used in the scientific publication analysis. There is the year of their first mention in the Top25 list and the consecutive mentions in the following Top25 lists. Species names are the current species names (based on IUCN) and not the name used at the time of the Top25 list release. First Second Third Fourth Fifth Sixth Species mention mention mention mention mention mention Ateles fusciceps 2006 Ateles hybridus 2006 2008 2010 Ateles hybridus brunneus 2004 Brachyteles hypoxanthus 2000 2002 2004 Callicebus barbarabrownae 2010 Cebus flavius 2010 Cebus xanthosternos 2000 2002 2004 Cercocebus atys lunulatus 2000 2002 2004 Cercocebus galeritus galeritus 2002 Cercocebus sanjei 2000 2002 2004 Cercopithecus roloway 2002 2006 2008 2010 Cercopithecus sclateri 2000 Eulemur cinereiceps 2004 2006 2008 Eulemur flavifrons 2008 2010 Galagoides rondoensis 2006 2008 2010 Gorilla beringei graueri 2010 Gorilla beringei beringei 2000 2002 2004 Gorilla gorilla diehli 2000 2002 2004 2006 2008 Hapalemur aureus 2000 Hapalemur griseus alaotrensis 2000 Hoolock hoolock 2006 2008 Hylobates moloch 2000 Lagothrix flavicauda 2000 2006 2008 2010 Leontopithecus caissara 2000 2002 2004 Leontopithecus chrysopygus 2000 Leontopithecus rosalia 2000 Lepilemur sahamalazensis 2006 Lepilemur septentrionalis 2008 2010 Loris tardigradus nycticeboides -

Laughing Gulls Breed Primarily Along the Pacific Coast of Mexico and the Atlantic and Caribbean Coasts from S
LAUGHING GULL Leucophaeus atricilla non-breeding visitor, regular winterer L.a. megalopterus Laughing Gulls breed primarily along the Pacific coast of Mexico and the Atlantic and Caribbean coasts from s. Canada to Venezuela, and they winter S to Peru and the Amazon delta (AOU 1998, Howell and Dunn 2007). It and Franklin's Gull were placed along with other gulls in the genus Larus until split by the AOU (2008). Vagrant Laughing Gulls have been reported in Europe (Cramp and Simmons 1983) and widely in the Pacific, from Clipperton I to Wake Atoll (Rauzon et al. 2008), Johnston Atoll (records of at least 14 individuals, 1964-2003), Palmyra, Baker, Kiribati, Pheonix, Marshall, Pitcairn, Gambier, and Samoan Is, as well as Australia/New Zealand (King 1967; Clapp and Sibley 1967; Sibley and McFarlane 1968; Pratt et al. 1987, 2010; Garrett 1987; Wragg 1994; Higgins and Davies 1996; Vanderwerf et al. 2004; Hayes et al. 2015; E 50:13 [identified as Franklin's Gull], 58:50). Another interesting record is of one photographed attending an observer rowing solo between San Francisco and Australia at 6.5° N, 155° W, about 1400 km S of Hawai'i I, 1-2 Nov 2007. They have been recorded almost annually as winter visitors to the Hawaiian Islands since the mid-1970s, numbers increasing from the NW to the SE, as would be expected of this N American species. The great majority of records involve first-year birds, and, despite the many records in the S Pacific, there is no evidence for a transient population through the Hawaiian Islands, or of individuals returning for consecutive winters after departing in spring. -

First Confirmed Record of Belcher's Gull Larus Belcheri for Colombia with Notes on the Status of Other Gull Species
First confirmed record of Belcher's Gull Larus belcheri for Colombia with notes on the status of other gull species Primer registro confirmado de la Gaviota Peruana Larus belcheri para Colombia con notas sobre el estado de otras especies de gaviotas Trevor Ellery1 & José Ferney Salgado2 1 Independent. Email: [email protected] 2 Corporación para el Fomento del Aviturismo en Colombia. Abstract We present photographic records of a Belcher's Gull Larus belcheri from the Colombian Caribbean region. These are the first confirmed records of this species in the country. Keywords: new record, range extension, gull, identification. Resumen Presentamos registros fotograficos de un individuo de la Gaviota Peruana Larus belcheri en la region del Caribe de Colombia. Estos son los primeros registros confirmados para el país. Palabras clave: Nuevo registro, extensión de distribución, gaviota, identificación. Introduction the Pacific Ocean coasts of southern South America, and Belcher's Gull or Band-tailed Gull Larus belcheri has long Olrog's Gull L. atlanticus of southern Brazil, Uruguay and been considered a possible or probable species for Argentina (Howell & Dunn 2007, Remsen et al. 2018). Colombia, with observations nearby from Panama (Hilty & Brown 1986). It was first listed for Colombia by Salaman A good rule of thumb for gulls in Colombia is that if it's not et al. (2001) without any justification or notes, perhaps on a Laughing Gull Leucophaeus atricilla, then it's interesting. the presumption that the species could never logically have A second good rule of thumb for Colombian gulls is that if reached the Panamanian observation locality from its it's not a Laughing Gull, you are probably watching it at Los southern breeding grounds without passing through the Camarones or Santuario de Fauna y Flora Los Flamencos, country. -

World's Most Endangered Primates
Primates in Peril The World’s 25 Most Endangered Primates 2016–2018 Edited by Christoph Schwitzer, Russell A. Mittermeier, Anthony B. Rylands, Federica Chiozza, Elizabeth A. Williamson, Elizabeth J. Macfie, Janette Wallis and Alison Cotton Illustrations by Stephen D. Nash IUCN SSC Primate Specialist Group (PSG) International Primatological Society (IPS) Conservation International (CI) Bristol Zoological Society (BZS) Published by: IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), Bristol Zoological Society (BZS) Copyright: ©2017 Conservation International All rights reserved. No part of this report may be reproduced in any form or by any means without permission in writing from the publisher. Inquiries to the publisher should be directed to the following address: Russell A. Mittermeier, Chair, IUCN SSC Primate Specialist Group, Conservation International, 2011 Crystal Drive, Suite 500, Arlington, VA 22202, USA. Citation (report): Schwitzer, C., Mittermeier, R.A., Rylands, A.B., Chiozza, F., Williamson, E.A., Macfie, E.J., Wallis, J. and Cotton, A. (eds.). 2017. Primates in Peril: The World’s 25 Most Endangered Primates 2016–2018. IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), and Bristol Zoological Society, Arlington, VA. 99 pp. Citation (species): Salmona, J., Patel, E.R., Chikhi, L. and Banks, M.A. 2017. Propithecus perrieri (Lavauden, 1931). In: C. Schwitzer, R.A. Mittermeier, A.B. Rylands, F. Chiozza, E.A. Williamson, E.J. Macfie, J. Wallis and A. Cotton (eds.), Primates in Peril: The World’s 25 Most Endangered Primates 2016–2018, pp. 40-43. IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), and Bristol Zoological Society, Arlington, VA. -

WAAVP2019-Abstract-Book.Pdf
27th Conference of the World Association for the Advancement of Veterinary Parasitology JULY 7 – 11, 2019 | MADISON, WI, USA Dedicated to the legacy of Professor Arlie C. Todd Sifting and Winnowing the Evidence in Veterinary Parasitology @WAAVP2019 @WAAVP_2019 Abstract Book Joint meeting with the 64th American Association of Veterinary Parasitologists Annual Meeting & the 63rd Annual Livestock Insect Workers Conference WAAVP2019 27th Conference of the World Association for the Advancements of Veterinary Parasitology 64th American Association of Veterinary Parasitologists Annual Meeting 1 63rd Annualwww.WAAVP2019.com Livestock Insect Workers Conference #WAAVP2019 Table of Contents Keynote Presentation 84-89 OA22 Molecular Tools II 89-92 OA23 Leishmania 4 Keynote Presentation Demystifying 92-97 OA24 Nematode Molecular Tools, One Health: Sifting and Winnowing Resistance II the Role of Veterinary Parasitology 97-101 OA25 IAFWP Symposium 101-104 OA26 Canine Helminths II 104-108 OA27 Epidemiology Plenary Lectures 108-111 OA28 Alternative Treatments for Parasites in Ruminants I 6-7 PL1.0 Evolving Approaches to Drug 111-113 OA29 Unusual Protozoa Discovery 114-116 OA30 IAFWP Symposium 8-9 PL2.0 Genes and Genomics in 116-118 OA31 Anthelmintic Resistance in Parasite Control Ruminants 10-11 PL3.0 Leishmaniasis, Leishvet and 119-122 OA32 Avian Parasites One Health 122-125 OA33 Equine Cyathostomes I 12-13 PL4.0 Veterinary Entomology: 125-128 OA34 Flies and Fly Control in Outbreak and Advancements Ruminants 128-131 OA35 Ruminant Trematodes I Oral Sessions -

Primate Conservation 2006 (20): 1–28
Contents General Primates in Peril: The World’s 25 Most Endangered Primates, 2004–2006 ..................................................................................1 Russell A. Mittermeier, Cláudio Valladares-Pádua, Anthony B. Rylands, Ardith A. Eudey, Thomas M. Butynski, Jörg U. Ganzhorn, Rebecca Kormos, John M. Aguiar and Sally Walker Neotropical Region On a New Species of Titi Monkey, Genus Callicebus Thomas (Primates, Pitheciidae), from Western Bolivia with Preliminary Notes on Distribution and Abundance ...............................................................................................................29 Robert. B. Wallace, Humberto Gómez, Annika Felton and Adam M. Felton Identifi cation, Behavioral Observations, and Notes on the Distribution of the Titi Monkeys Callicebus modestus Lönnberg, 1939 and Callicebus olallae Lönnberg, 1939 ..............................................................................41 Adam Felton, Annika M. Felton, Robert B. Wallace and Humberto Gómez A Survey of Primate Populations in Northeastern Venezuelan Guayana .....................................................................................47 Bernardo Urbani A History of Long-term Research and Conservation of Northern Muriquis (Brachyteles hypoxanthus) at the Estação Biológica de Caratinga/RPPN-FMA .......................................................................................................................53 Karen B. Strier and Jean Philippe Boubli Africa English Common Names for Subspecies and Species of African Primates