The Local Chemical Environment of Nodes of Ranvier: a Study of Cation Binding
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
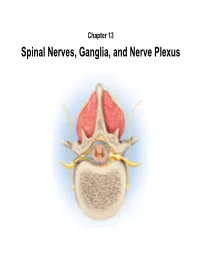
Spinal Nerves, Ganglia, and Nerve Plexus Spinal Nerves
Chapter 13 Spinal Nerves, Ganglia, and Nerve Plexus Spinal Nerves Posterior Spinous process of vertebra Posterior root Deep muscles of back Posterior ramus Spinal cord Transverse process of vertebra Posterior root ganglion Spinal nerve Anterior ramus Meningeal branch Communicating rami Anterior root Vertebral body Sympathetic ganglion Anterior General Anatomy of Nerves and Ganglia • Spinal cord communicates with the rest of the body by way of spinal nerves • nerve = a cordlike organ composed of numerous nerve fibers (axons) bound together by connective tissue – mixed nerves contain both afferent (sensory) and efferent (motor) fibers – composed of thousands of fibers carrying currents in opposite directions Anatomy of a Nerve Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Epineurium Perineurium Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Endoneurium Nerve Rootlets fiber Posterior root Fascicle Posterior root ganglion Anterior Blood root vessels Spinal nerve (b) Copyright by R.G. Kessel and R.H. Kardon, Tissues and Organs: A Text-Atlas of Scanning Electron Microscopy, 1979, W.H. Freeman, All rights reserved Blood vessels Fascicle Epineurium Perineurium Unmyelinated nerve fibers Myelinated nerve fibers (a) Endoneurium Myelin General Anatomy of Nerves and Ganglia • nerves of peripheral nervous system are ensheathed in Schwann cells – forms neurilemma and often a myelin sheath around the axon – external to neurilemma, each fiber is surrounded by -
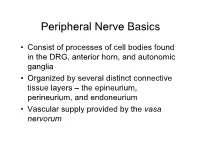
PN1 (Midha) Microanatomy of Peripheral Nerves-Part1.Pdf
Peripheral Nerve Basics • Consist of processes of cell bodies found in the DRG, anterior horn, and autonomic ganglia • Organized by several distinct connective tissue layers – the epineurium, perineurium, and endoneurium • Vascular supply provided by the vasa nervorum Peripheral Nerve Basics • Neuronal processes bound into fascicles by perineurium • Fascicles bound into nerves by epineurium • Endoneurium is a division of the perineurium which form thin layers of connective tissue surrounding neuronal fibers in a fascicle Sural nerve in cross-section Epineurium • Loose areolar tissue with sparse, longitudinally-oriented collagen fibers • Some elastic fibers where epineurium abuts perineurium • Able to accommodate a significant amount of nerve stretching and movement • Increases in thickness where nerves cross joints • Constitutes an increasing proportion of nerves as they increase in size • Epineurial fat helps cushion nerves from compressive injury • Decreased epineurial fat found in patients with diabetes Perineurium • Cellular component composed of laminated fibroblasts of up to 15 layers in thickness which are bounded by a basal lamina • Semi-permeable: inner lamellae have tight junctions, providing a barrier to intercellular transport of macromolecules – Tight junctions can be loosened with topical anaesthetics and with osmotic change Perineurium • Exhibits a slightly positive internal pressure – Fascicular contents herniate upon perineurial injury • Under tension longitudinally – Nerve segment shortens upon transection – may complicate surgical repair as nerve can be stretched only approximately 10% before being inhibited by collagen Endoneurium • Intrafascicular connective tissue consisting of a collagenous matrix in the interstitial space • Develops into partitions of dense connective tissue between diverging fascicles and eventually becomes perineurium when the fascicles separate • Collagen fibers are longitudinally-oriented and run along nerve fibers and capillaries. -

Nerve and Nerve Injuries” Sunderland : 50 Years Later
2019 Nerve and Nerve Injuries” Sunderland : 50 years later Faye Chiou Tan, MD Professor, Dir. EDX, H. Ben Taub PMR, Baylor College of Medicine Chief PMR, Dir. EDX, Harris Health System 2019 Financial Disclosure • Elsevier Book Royalties for “EMG Secrets” textbook • Revance, consultation panel 2019 Warning Videotaping or taking pictures of the slides associated with this presentation is prohibited. The information on the slides is copyrighted and cannot be used without permission and author attribution. Introduction – Sydney Sunderland was Professor of Experimental Neurology at the University of Melbourne. – His textbook “Nerve and Nerve lnjuries” published in 1968 is no longer in print (copies $1000 on the internet) – Here is a review as relates to new technology: Ultrahigh frequency musculoskeletal ultrasound Part I – I. Anatomic and physiologic features of A. Peripheral nerve fibers B. Peripheral nerve trunks I.A. Peripheral nerve fibers – Axoplasm – Increased flow of cytoplasm from cell body into axons during electrical stimulation (Grande and Richter 1950) – Although overall proximal to distal axoplasmic flow, the pattern of streaming in the axon is bidirectional and faster (up to 3-7 cm/day) (Lubinska 1964). I. A. Peripheral nerve fibers – Sheath – Myelinated – Length of internode elongates with growth (Vizoso and Young 1948, Siminoff 1965) – In contrast, remyelination in adults produce short internodes of same length (Leegarrd 1880, Young 1945,…) – Incisures of Schmidt-Lantermann are clefts conical clefts that open when a nerve trunk is stretched thereby preventing distortion of myelin. (Glees, 1943) Schmidt-Lantermann Clefts Sunderland S. Nerve and Nerve Injuries, Sunderland, Livingstone,LTD, Edinburgh/London, 1968, p. 8 I. A. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

The “Road Map”
PRACTICAL ROADMAP NERVOUS TISSUE DR N GRAVETT NEURONS • MOTOR • SENSORY Anterior (ventral) horn Dorsal root of spinal of spinal cord cord Multipolar Pseudounipolar ANTERIOR HORN CELLS • Slide 64 Spinal Cord (vervet monkey) Stain: Kluver and Berrera Technique NOTE: with this technique, myelin stains dark blue and basophilic substances such as rER and nuclei stain violet. In this case we use “blue” and “purple” to describe the staining and not eosinophilic and basophilic. SPINAL CORD Anterior Ventral Horn Arachnoid Ventricle Pia Mater Grey Matter White Matter Posterior Horn Dura Mater Dorsal ANTERIOR HORN CELL Neuropil Cell Body Dendrite Vesicular Nucleus Nucleolus Nucleus of Nissl Bodies Neuroglial Cell ANTERIOR HORN CELL Neuropil Cell Body Vesicular Nucleus Nucleolus Nissl Body Nucleus of Neuroglial Cell Dendrite Nissl Body Axon Hillock Axon SPINAL (DORSAL ROOT) GANGLION CELLS • Slide 62 Spinal Ganglion Stain: H&E NOTE: The spinal ganglion is also known as the dorsal root ganglia and contains pseudounipolar neuron cell bodies. SPINAL (DORSAL ROOT) GANGLIA Cell Bodies Processes (Axons and Dendrites) SPINAL (DORSAL ROOT) GANGLIA Cell Bodies Processes (Axons and Dendrites) NOTE: The neuronal cell bodies of the dorsal root ganglia are “clumped” together, and one cannot see any processes entering or leaving the cell bodies. The processes (axons and dendrites) are seen towards the edge/periphery of the group of cell bodies. SPINAL (DORSAL ROOT) GANGLIA Satellite cells (arranged in ring like fashion around the cell body) Cell Body Nucleolus Vesicular Fine Granular Nucleus Nissl Substance Nucleus of Satellite cell PERIPHERAL BRANCH OF A SPINAL NERVE • Slide 32 Median Nerve Stain: Mallory’s Technique NOTE: Three dyes are used in Mallory’s technique, which results in collagen fibres (such as connective tissue) staining blue, the “neurokeratin” staining red, and nuclei staining reddish-orange PERIPHERAL NERVE Myelinated Axons Vein L.S. -

School of Physical Education and Sports Science
ARISTOTELIAN UNIVERSITY OF THESSALONIKI School of Physical Education and Sports Science Bachelor's Thesis: "MYOFASCIAL NETWORK IN PHYSICAL ACTIVITY AND TRAINING" Name: Tsourvakas Konstantinos Supervisor: Papadopoulos Panagiotis PhD Thessaloniki, June 2018 1 ABSTRACT Physical activity can lead people, regardless of age, to a healthier and more quality life. Especially nowadays, that most people follow an intense routine, good physical condition is an essential factor to take care of in order to achieve health and well- being. This project refers to the myofascial system of the human body. It is about a scientific review whose purpose is to highlight the importance of self myofascial release (SMR), to quote the process of SMR through the use of specific tools and methods, and the analysis of its effects to the human body. In the first place, the fascial network of the human body is described, along with the properties, the function and the usefulness of the fascia. Subsequently, the assignment focuses on the significance of self myofascial release for the human body and mostly the way it increases and improves the range of motion. In addition, there is a presentation of SMR's techniques and methods with the application of tools such as foam rollers, roller massagers and tennis balls. The assignment ends up with conclusions and suggestions on further improvement of range of motion. 2 TABLE OF CONTENTS Abstract……………………………………………………………………….………2 Table of Contents ……………………….......………………………………………...3 1. Introduction...………………………………………………………………..……...4 2. The Fascial System………………………………………………..………………..5 2.1 Definition….…………………………………………………..…………………..5 2.2 Structure….……………………………………………………………..…………5 2.3 Function………………………………………………………………..…………22 3. Importance of training fascia …………….....……………………………………..27 3.1 Principles of training fascia....................................................................................27 3.2 Fascia and elastic recoil.........................................................................................31 4. -

Lecture 19: Nerve Synthesis in Vivo (Regeneration)
Nerve synthesis in vivo (regeneration)* 1. Anatomy and function of a peripheral nerve. 2. Experimental parameters for study of induced regeneration. 3. Synthesis of myelinated axons and BM (nerve fibers) 4. Evidence (?) of synthesis of an endoneurium. 5. Synthesis of a nerve trunk (including summary of kinetics of synthesis). 6. Comparative regenerative activity of various reactants. _______ *Tissue and Organ Regeneration in Adults, Yannas, Springer, 2001, Ch. 6. 1. Anatomy and function of a peripheral nerve. I Nervous system = central nervous system (CNS) + peripheral nervous system (PNS) Image: public domain (by Wikipedia User: Persion Poet Gal) Nervous System: CNS and PNS CNS PNS Chamberlain, Yannas, et al., 1998 Landstrom, Aria. “Nerve Regeneration Induced by Collagen-GAG Matrix in Collagen Tubes.” MS Thesis, MIT, 1994. Focus of interest: nerve fibers and axons Nerve fibers comprise axons wrapped in a myelin sheath, itself surrounded by BM (diam. 10-30 μm in rat sciatic nerve). Axons are extensions (long processes) of neurons located in spinal cord. They comprise endoplasmic reticulum and microtubules. 1. Anatomy and function of a peripheral nerve. II Myelinated axons (diam. 1-15 μm) are wrapped in a myelin sheath; nonmyelinated axons also exist. They are the elementary units for conduction of electric signals in the body. Myelin formed by wrapping a Schwann cell membrane many times around axon perimeter. No ECM inside nerve fibers. Myelin sheath is a wrapping of Schwann cell membranes around certain axons. 1. Anatomy and function of a peripheral nerve. III Nonmyelinated axons (diam. <1 μm) function in small pain nerves. Although surrounded by Schwann cells, they lack myelin sheath; Schwann cells are around them but have retained their cytoplasm. -

Terminology List for the Nervous System
Terminology List for the Nervous System Nerve cell: neuron, cell body, axon, dendrite, axon hillock, axon terminal, synaptic cleft, neuroglia, Schwann cells, myelin, Nodes of Ranvier, neurilemma, endoneurium Human Brain: cerebrum: right and left cerebral hemispheres transverse fissure longitudinal fissure lateral sulcus central sulcus parieto-occipital sulcus precentral gyrus postcentral gyrus frontal lobe parietal lobe temporal lobe occipital lobe insula cortex basal nuclei (=cerebral nuclei or basal ganglia) corpus callosum septum pellucidum fornix internal capsule cerebellum: right and left cerebellar hemispheres vermis cortex arbor vitae diencephalon: epithalamus (pineal body, pineal gland) thalamus intermediate mass hypothalamus infundibulum pituitary gland mammillary bodies brainstem: midbrain (=mesencephalon) corpora quadrigemina superior colliculi inferior colliculi cerebral peduncles pons cerebellar peduncles medulla oblongata pyramids Sheep Brain: Cerebrum: cerebral hemispheres, gyri, sulci, olfactory bulbs, olfactory tracts, optic nerves, optic chiasma, corpus callosum Diencephalon: epithalamus (or pineal gland), thalamus hypothalamus, pituitary gland Cerebellum: arbor vitae Brain Stem: midbrain pons medulla Meninges & Ventricles: dura mater, arachnoid layer, subacrachnoid space, pia mater, falx cerebri, falx cerebelli, tentorium cerebelli, lateral ventricles, third ventricle, cerebral aqueduct, fourth ventricle, choroid plexuses, arachnoid villi (=arachnoid granulations) Spinal cord: central canal, posterior median sulcus, -
Nervous System -I
Body Tissues Epithelial Connective Tissues Muscle Nervous Nervous system Controlling & Coordinating System Conducts nerve impulses between body structures and controls body functions Functions • Sensory Internal External • Integration> Analysis> storage>interpret>decide • Motor> Response • Regulates all activity (Voluntary & Involuntary) • Adjust according to changing external and internal environment Nervous System Subdivisions: CNS (Central Nervous System) PNS( Peripheral Nervous System) ANS (Autonomic Nervous system) Nervous tissue - Cell Types Functionally • Neuron (Nerve Cell) -Conduction Variable Shape , Size, Function • Neuroglia - Supportive -- Macroglia -- Microglia • Ependymal Cells • Schwann Cells - In PNS Neuron ( Nerve Cell) Components 1.Cell Body 2.Cell Processes (Neurites) Cell Body - Size vary from 5 µm - 120 µm (Perikaryon) – Plasma membrane Nucleus Cytoplasm Axon Hillock Neuronal Skeleton Cell Processes 1.Dendrites : Short , irregular thickness. Freely Branching, Afferent processes , Contain Nissl Granules 2. Axon – Long , Single, Efferent process of Uniform Diameter, Devoid of Nissl Granules, Ensheathed by Schwann cells, Gives collateral branches Terminal branches called telodendria (axon terminals) Terminate – within CNS - Always with another neuron Outside CNS – Either may end in relation to the effector organ or Synapse with neurons of Peripheral ganglia Types Of Neuron 1.Acc. To no of Processes Bipolar Multipolar Pseudounipolar 2. Acc. To Function Sensory Motor 3. Acc. To Axon Length Golgi type-1(long) Golgi type-II Synapse site of junction of neuron Types Axo- Dendritic Axo – Somatic Axo- Axonal Neuroglia • Astrocytes : Fibrous Protoplasmic Metabolism of neurotransmitters K+ Balance Contribute in brain development Blood brain barrier Link between neurons and blood vessels • Oligodendrocytes: Form a supporting network around neurons Produce myelin sheath around several neurons Neuroglia- contd. • Microglia: Phagocytic cells; Migrate to area of injured nervous tissue. -

Perineurial Glia
Downloaded from http://cshperspectives.cshlp.org/ on September 23, 2021 - Published by Cold Spring Harbor Laboratory Press Perineurial Glia Sarah Kucenas Department of Biology, University of Virginia, Charlottesville, Virginia 22904 Correspondence: [email protected] Although the ultrastructure of peripheral nerves has been known for nearly 200 years, the developmental origins and functional roles of all five main components of these specialized nervous system conduits are still poorly understood. One of these understudied nerve ele- ments, the perineurium, is a component of the blood–nerve barrier and is essential for protecting axons and their associated Schwann cells from ionic flux, toxins, and infection. However, until recently, it was thought that this vital nerve tissue was derived from the mesoderm and simply served a structural/barrier function with no other influence on the development, maintenance, or regeneration of peripheral nerves. Recent work in zebrafish using in vivo time-lapse imaging, genetic manipulation, and laser axotomy is shedding light on the origin and roles of this previously ignored glial nerve component and is changing how we view development of the nervous system. ll nerves of the peripheral nervous system linated, whereas smaller diameter axons are of- A(PNS) are structurally identical, consist- ten associated within nonmyelinating Schwann ing of five main components (Fig. 1) (Gamble cells known as Remak bundles (Fig. 1). These and Eames 1964; Cravioto 1965; Shantha and axon–Schwann cell complexes reside within Bourne 1968; Akert et al. 1976; Nordlander et the endoneurium, a neural crest–derived tissue, al.1981). Centralto all sensoryand motor nerves which synthesizes and secrets extracellular ma- are axons. -

The Neural Basis of Speech and Language
© Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION CHAPTER © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC The NeuralNOT FOR SALE Basis OR DISTRIBUTION of NOT FOR SALE OR DISTRIBUTION Speech and Language 2 © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Introduction © Jones & BartlettThis section Learning, gives the LLC reader a brief overview ©of Joneswhat takes & Bartlett place neurally Learning, when LLCa per- son starts a conversation by saying, “Hello. How are you? How was your vacation NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION trip?” to another individual whom the person meets on the street. Simply put, the steps involved would be as follows: 1. Basic vision: seeing a person on the street 2. Visual perception: recognizing the person as someone the speaker knows 3. Cognition:© theJones desire & to Bartlett speak with Learning, this person LLC about a trip that the speaker© Jones may & Bartlett Learning, LLC want to takeNOT in FORthe future SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION 4. Language: searching for the right sounds, syllables, words, and sentences, all pre- sented in the right order, with meaning properly related to the greeting and the subject matter, to be expressed with a positive attitude © Jones5. Motor & Bartlettprogramming Learning, or planning: LLC readying the speech© mechanism Jones & Bartlettjust prior Learning, to LLC NOT speakingFOR SALE so that OR the DISTRIBUTION production is correct NOT FOR SALE OR DISTRIBUTION 6. Motor production or execution: speaking 7. -

26 April 2010 TE Prepublication Page 1 Nomina Generalia General Terms
26 April 2010 TE PrePublication Page 1 Nomina generalia General terms E1.0.0.0.0.0.1 Modus reproductionis Reproductive mode E1.0.0.0.0.0.2 Reproductio sexualis Sexual reproduction E1.0.0.0.0.0.3 Viviparitas Viviparity E1.0.0.0.0.0.4 Heterogamia Heterogamy E1.0.0.0.0.0.5 Endogamia Endogamy E1.0.0.0.0.0.6 Sequentia reproductionis Reproductive sequence E1.0.0.0.0.0.7 Ovulatio Ovulation E1.0.0.0.0.0.8 Erectio Erection E1.0.0.0.0.0.9 Coitus Coitus; Sexual intercourse E1.0.0.0.0.0.10 Ejaculatio1 Ejaculation E1.0.0.0.0.0.11 Emissio Emission E1.0.0.0.0.0.12 Ejaculatio vera Ejaculation proper E1.0.0.0.0.0.13 Semen Semen; Ejaculate E1.0.0.0.0.0.14 Inseminatio Insemination E1.0.0.0.0.0.15 Fertilisatio Fertilization E1.0.0.0.0.0.16 Fecundatio Fecundation; Impregnation E1.0.0.0.0.0.17 Superfecundatio Superfecundation E1.0.0.0.0.0.18 Superimpregnatio Superimpregnation E1.0.0.0.0.0.19 Superfetatio Superfetation E1.0.0.0.0.0.20 Ontogenesis Ontogeny E1.0.0.0.0.0.21 Ontogenesis praenatalis Prenatal ontogeny E1.0.0.0.0.0.22 Tempus praenatale; Tempus gestationis Prenatal period; Gestation period E1.0.0.0.0.0.23 Vita praenatalis Prenatal life E1.0.0.0.0.0.24 Vita intrauterina Intra-uterine life E1.0.0.0.0.0.25 Embryogenesis2 Embryogenesis; Embryogeny E1.0.0.0.0.0.26 Fetogenesis3 Fetogenesis E1.0.0.0.0.0.27 Tempus natale Birth period E1.0.0.0.0.0.28 Ontogenesis postnatalis Postnatal ontogeny E1.0.0.0.0.0.29 Vita postnatalis Postnatal life E1.0.1.0.0.0.1 Mensurae embryonicae et fetales4 Embryonic and fetal measurements E1.0.1.0.0.0.2 Aetas a fecundatione5 Fertilization