Genetic Variation, Biochemical Contents and Wound Healing Activity of Plantago Major
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Central Composite Design for Optimizing the Biosynthesis Of
www.nature.com/scientificreports OPEN Central Composite Design for Optimizing the Biosynthesis of Silver Nanoparticles using Plantago major Extract and Investigating Antibacterial, Antifungal and Antioxidant Activity Ghazal Nikaeen1, Saeed Yousefnejad1 ✉ , Samane Rahmdel2, Fayezeh Samari3 & Saeideh Mahdavinia1 Central composite design (CCD) was applied to optimize the synthesis condition of silver nanoparticles (AgNPs) using the extract of Plantago major (P. major) seeds via a low cost and single-step process. The aqueous seed extract was applied as both reducing element and capping reagent for green production of AgNPs. Five empirical factors of synthesis including temperature (Temp), pH, volume of P. major extract (Vex), volume of AgNO3 solution (VAg) and synthesis time were used as independent variables of model and peak intensity of Surface Plasmon Resonance (SPR) originated from NPs as the dependent variable. The predicted optimal conditions was determined to be: Temp = 55 °C, pH = 9.9,Vex = 1.5 mL, VAg = 30 mL, time = 60 min. The characterization of the prepared AgNPs at these optimum conditions was conducted by Fourier transform infrared spectroscopy (FTIR), dynamic light scattering (DLS), transmission electron microscopy (TEM) and X-ray difraction (XRD) to determine the surface bio- functionalities. Bio-activity of these AgNPs against bacteria and fungi were evaluated based on its assay against Micrococcus luteus, Escherichia coli and Penicillium digitatum. Furthermore, antioxidant capacity of these NPs was checked using the ferric reducing antioxidant power (FRAP) assay. Nanotechnology is an important feld of modern research which has been the principal of various technologies and main innovations; and is expected to be the basis of many other outstanding innovations in future. -

Anthracene Derivatives in Some Species of Rumex L
Vol. 76, No. 2: 103-108, 2007 ACTA SOCIETATIS BOTANICORUM POLONIAE 103 ANTHRACENE DERIVATIVES IN SOME SPECIES OF RUMEX L. GENUS MAGDALENA WEGIERA1, HELENA D. SMOLARZ1, DOROTA WIANOWSKA2, ANDRZEJ L. DAWIDOWICZ2 1 Departament of Pharmaceutical Botany Skubiszewski Medical University of Lublin Chodki 1, 20-039 Lublin, Poland e-mail: [email protected] 2 Faculty of Chemistry, Marii Curie-Sk³odowska University of Lublin (Received: June 1, 2006. Accepted: September 5, 2006) ABSTRACT Eight anthracene derivatives (chrysophanol, physcion, emodin, aloe-emodin, rhein, barbaloin, sennoside A and sennoside B) were signified in six species of Rumex L genus: R. acetosa L., R. acetosella L., R. confertus Willd., R. crispus L., R. hydrolapathum Huds. and R. obtusifolius L. For the investigations methanolic extracts were pre- pared from the roots, leaves and fruits of these species. Reverse Phase High Performance Liquid Chromatography was applied for separation, identification and quantitative determination of anthracene derivatives. The identity of these compounds was further confirmed with UV-VIS. Received data were compared. The roots are the best organs for the accumulation of anthraquinones. The total amount of the detected compo- unds was the largest in the roots of R. confertus (163.42 mg/g), smaller in roots R. crispus (25.22 mg/g) and the smallest in roots of R. hydrolapathum (1.02 mg/g). KEY WORDS: Rumex sp., roots, fruits, leaves, anthracene derivatives, RP-HPLC. INTRODUCTION scion-anthrone, rhein, nepodin, nepodin-O-b-D-glycoside and 1,8-dihydroxyanthraquinone from R. acetosa (Dedio The species belonging to the Rumex L. genus are wide- 1973; Demirezer and Kuruuzum 1997; Fairbairn and El- spread in the world. -
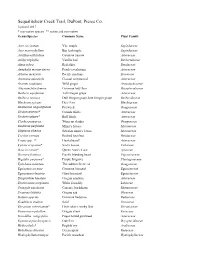
Sequalitchew Creek Trail Plant List
Sequalitchew Creek Trail, DuPont, Pierce Co. Updated 2017 * non-native species ** native and non-native Genus/Species Common Name Plant Family Acer circinatum Vine maple Sapindaceae Acer macrophyllum Big leaf maple Sapindaceae Achillea millefolium Common yarrow Asteraceae Achlys triphylla Vanilla leaf Berberidaceae Alnus rubra Red alder Betulaceae Anaphalis margaritacea Pearly everlasting Asteraceae Arbutus menziesii Pacific madrone Ericaceae Artemisia suksdorfii Coastal wormwood Asteraceae Asarum caudatum Wild ginger Aristolochiaceae Athyrium felix-femina Common lady fern Dryopteridaceae Berberis aquifolium Tall Oregon grape Asteraceae Berberis nervosa Dull Oregon grape, low Oregon grape Berberidaceae Blechnum spicant Deer fern Blechnaceae Chamerion angustifolium Fireweed Onagraceae Cirsium arvense* Canada thistle Asteraceae Cirsium vulgare* Bull thistle Asteraceae Clarkia purpurea Winecup clarkia Onagraceae Claytonia perfoliata Miner's lettuce Montiaceae Claytonia siberica Siberian miner's lettuce Montiaceae Corylus cornuta Beaked hazelnut Betulaceae Crepis spp. ?* Hawksbeard? Asteraceae Cytisus scoparius* Scot's broom Fabaceae Daucus carota* Queen Anne's Lace Apiaceae Dicentra formosa Pacific bleeding heart Papaveraceae Digitalis purpurea* Purple foxglove Plantaginaceae Epilobium minutum Threadstem fireweed Onagraceae Equisetum arvense Common horsetail Equisetaceae Equisetum telmateia Giant horsetail Equisetaceae Eriophyllum lanatum Oregon sunshine Asteraceae Erythronium oregonum White fawn lily Liliaceae Frangula purshiana Cascara, -

Concentrations of Anthraquinone Glycosides of Rumex Crispus During Different Vegetation Stages L
Concentrations of Anthraquinone Glycosides of Rumex crispus during Different Vegetation Stages L. Ömtir Demirezer Hacettepe University, Faculty of Pharmacy, Department of Pharmacognosy, 06100 Ankara, Turkey Z. Naturforsch. 49c, 404-406 (1994); received January 31, 1994 Rumex crispus, Polygonaceae. Anthraquinone, Glycoside The anthraquinone glycoside contents of various parts of Rumex crispus L. (Polygonaceae) in different vegetation stages were investigated by thin layer chromatographic and spectro- photometric methods. The data showed that the percentage of anthraquinone glycoside in all parts of plant increased at each stage. Anthraquinone glycoside content was increased in leaf, stem, fruit and root from 0.05 to 0.40%. from 0.03 to 0.46%. from 0.08 to 0.34%, and from 0.35 to 0.91% respectively. From the roots of R. crispus, emodin- 8 -glucoside, RGA (isolated in our laboratory, its structure was not elucidated), traceable amount of glucofran- gulin B and an unknown glycoside ( R f = 0.28 in ethyl acetate:methanol:water/100:20:10) was detected in which the concentration was increased from May to August. The other parts of plant contained only emodin- 8 -glucoside. Introduction In the present investigation various parts of Rumex L. (Polygonaceae) is one of several Rumex crispus, leaf, stem, fruit and root were ana genera which is characterized by the presence lyzed separately for their anthraquinone glycoside of anthraquinone derivatives. There are about contents, the glycosides in different vegetation 200 species of Rumex in worldwide (Hegi, 1957). stages were detected individually. By this method, Rumex is represented with 23 species and 5 hy translocation of anthraquinone glycosides were brids in Turkey (Davis, 1965) and their roots have also investigated. -

Fort Ord Natural Reserve Plant List
UCSC Fort Ord Natural Reserve Plants Below is the most recently updated plant list for UCSC Fort Ord Natural Reserve. * non-native taxon ? presence in question Listed Species Information: CNPS Listed - as designated by the California Rare Plant Ranks (formerly known as CNPS Lists). More information at http://www.cnps.org/cnps/rareplants/ranking.php Cal IPC Listed - an inventory that categorizes exotic and invasive plants as High, Moderate, or Limited, reflecting the level of each species' negative ecological impact in California. More information at http://www.cal-ipc.org More information about Federal and State threatened and endangered species listings can be found at https://www.fws.gov/endangered/ (US) and http://www.dfg.ca.gov/wildlife/nongame/ t_e_spp/ (CA). FAMILY NAME SCIENTIFIC NAME COMMON NAME LISTED Ferns AZOLLACEAE - Mosquito Fern American water fern, mosquito fern, Family Azolla filiculoides ? Mosquito fern, Pacific mosquitofern DENNSTAEDTIACEAE - Bracken Hairy brackenfern, Western bracken Family Pteridium aquilinum var. pubescens fern DRYOPTERIDACEAE - Shield or California wood fern, Coastal wood wood fern family Dryopteris arguta fern, Shield fern Common horsetail rush, Common horsetail, field horsetail, Field EQUISETACEAE - Horsetail Family Equisetum arvense horsetail Equisetum telmateia ssp. braunii Giant horse tail, Giant horsetail Pentagramma triangularis ssp. PTERIDACEAE - Brake Family triangularis Gold back fern Gymnosperms CUPRESSACEAE - Cypress Family Hesperocyparis macrocarpa Monterey cypress CNPS - 1B.2, Cal IPC -

Agave Americana
Agave americana Agave americana, common names sentry plant, century plant, maguey or American aloe, is a species of flowering plant in the family Agavaceae, native to Mexico, and the United States in New Mexico, Arizona and Texas. Today, it is cultivated worldwide as an ornamental plant. It has become naturalized in many regions, including the West Indies, parts of South America, the southern Mediterranean Basin, and parts of Africa, India, China, Thailand, and Australia. Despite the common name "American aloe", it is not closely related to plants in the genus Aloe. Description Although it is called the century plant, it typically lives only 10 to 30 years. It has a spread around 6–10 ft (1.8–3.0 m) with gray-green leaves of 3–5 ft (0.9–1.5 m) long, each with a prickly margin and a heavy spike at the tip that can pierce deeply. Near the end of its life, the plant sends up a tall, branched stalk, laden with yellow blossoms, that may reach a total height up to 25–30 ft (8– 9 m) tall. Its common name derives from its semelparous nature of flowering only once at the end of its long life. The plant dies after flowering, but produces suckers or adventitious shootsfrom the base, which continue its growth. Taxonomy and naming A. americana was one of the many species described by Carl Linnaeus in the 1753 edition of Species Plantarum, with the binomial name that is still used today. Cultivation A. americana is cultivated as an ornamental plant for the large dramatic form of mature plants—for modernist, drought tolerant, and desert-style cactus gardens—among many planted settings. -

Concentrations of Anthraquinone Glycosides of Rumex Crispus During Different Vegetation Stages L
Concentrations of Anthraquinone Glycosides of Rumex crispus during Different Vegetation Stages L. Ömtir Demirezer Hacettepe University, Faculty of Pharmacy, Department of Pharmacognosy, 06100 Ankara, Turkey Z. Naturforsch. 49c, 404-406 (1994); received January 31, 1994 Rumex crispus, Polygonaceae. Anthraquinone, Glycoside The anthraquinone glycoside contents of various parts of Rumex crispus L. (Polygonaceae) in different vegetation stages were investigated by thin layer chromatographic and spectro- photometric methods. The data showed that the percentage of anthraquinone glycoside in all parts of plant increased at each stage. Anthraquinone glycoside content was increased in leaf, stem, fruit and root from 0.05 to 0.40%. from 0.03 to 0.46%. from 0.08 to 0.34%, and from 0.35 to 0.91% respectively. From the roots of R. crispus, emodin- 8 -glucoside, RGA (isolated in our laboratory, its structure was not elucidated), traceable amount of glucofran- gulin B and an unknown glycoside ( R f = 0.28 in ethyl acetate:methanol:water/100:20:10) was detected in which the concentration was increased from May to August. The other parts of plant contained only emodin- 8 -glucoside. Introduction In the present investigation various parts of Rumex L. (Polygonaceae) is one of several Rumex crispus, leaf, stem, fruit and root were ana genera which is characterized by the presence lyzed separately for their anthraquinone glycoside of anthraquinone derivatives. There are about contents, the glycosides in different vegetation 200 species of Rumex in worldwide (Hegi, 1957). stages were detected individually. By this method, Rumex is represented with 23 species and 5 hy translocation of anthraquinone glycosides were brids in Turkey (Davis, 1965) and their roots have also investigated. -

Agave Americana (Century Plant) Size/Shape
Agave americana (Century plant) Agave americana is a monocarpic plant. It flowers once after 10 years or more, reaching a height of 6 meters. The plant dies after blooming. Landscape Information French Name: Agave Américain Pronounciation: a-GAH-vee a-mer-ih-KAY-na Plant Type: Cactus / Succulent Origin: North America Heat Zones: 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 Hardiness Zones: 8, 9, 10, 11, 12 Uses: Specimen, Mass Planting, Container Size/Shape Growth Rate: Slow Tree Shape: Round Canopy Density: Medium Canopy Texture: Coarse Height at Maturity: 1.5 to 3 m Spread at Maturity: 1.5 to 3 meters Time to Ultimate Height: 10 to 20 Years Companion Plants: Lavandula spp., Yucca, Penstemon, Aloe vera Notes Landscape Design Advice: The plant is typically used in residences as a free-standing specimen, not planted in mass. However larger commercial Plant Image landscapes have room for mass plantings which can create a dramatic impact. Locate it at least 2 meters away from walks and other areas where people could contact the spiny foliage. Agave americana (Century plant) Botanical Description Foliage Leaf Arrangement: Alternate Leaf Venation: Nearly Invisible Leaf Persistance: Evergreen Leaf Type: Simple Leaf Blade: Over 80 cm Leaf Shape: Linear Leaf Margins: Spiny Leaf Textures: Rough Leaf Scent: No Fragance Color(growing season): Blue-Green Flower Flower Showiness: True Flower Size Range: Over 20 Flower Type: Spike Flower Sexuality: Monoecious (Bisexual) Flower Scent: Pleasant Flower Color: Yellow, White Seasons: Summer Trunk Trunk Susceptibility -

Aloe Vera Gel 8001-97-6
• i SUMMARY OF DATA FOR CHEMICAL SELECTION Aloe Vera Gel 8001-97-6 BASIS OF NOMINATION TO THE NTP Aloe vera is presented to the CSWG as a widely used cosmetic,food additive, and dietary supplement that results in exposure to adults, children, and the elderly. Naturally occurring aloe contains 1,8 dihydroxyanthracene derivatives that are known mutagens and that cause a laxative effect. when aloe products are consumed orally. However, most aloe products sold for oral consumption in the over . the-counter dietary supplement market have reduced quantities of 1,8-dihydroxyanthracenes. Because ofthe part ofthe aloe plant used, aloe vera gel has an especially low concentration of 1,8 dihydroxyanthracenes. The wound healing properties ofaloe have been considered "common knowledge" for thousands of years. However, it is only with recent techniques that these properties have been shown scientifically. These recent studies also raise questions about the ability ofaloe products to cause a proliferative effect on the cell, a process associated with a greater risk for carcinogenicity. Thus, aloe vera gel is recommended for a specialized dermal study to clarify if aloe products may be promoters ifadministered after initiation with a carcinogen. SELECTION STATUS ACTION BY CSWG: 12/14/98 Studies reguested: - Cell transformation assay - Mechanistic studies ofcancer promotion using TGAC mouse model - Use TPA and aloin as positive controls Priority: High Rationale/Remarks: - Widespread oral and dermal exposure to humans - Suspicion ofcarcinogenicity based -

Estrogen and Thyroid Hormone Receptor Activation by Medicinal Plants from Bahia, Brazil
medicines Article Estrogen and Thyroid Hormone Receptor Activation by Medicinal Plants from Bahia, Brazil Luã Tainã Costa Reis 1 ID , Magnus Régios Dias da Silva 2, Silvia Lima Costa 3, Eudes da Silva Velozo 4, Ronan Batista 5 ID and Suzana Telles da Cunha Lima 1,* ID 1 Laboratory of Bioprospection and Biotechnology (LaBBiotec), Institute of Biology, Federal University of Bahia (UFBA), Barão de Jeremoabo Street, 147-Ondina, Salvador, BA 40170-115, Brazil; [email protected] 2 Laboratory of Molecular and Translational Endocrinology, Department of Medicine, Federal University of São Paulo (UNIFESP), R. Sena Madureira, 1500-Vila Clementino, São Paulo, SP 04021-001, Brazil; [email protected] 3 Laboratory of Neurochemistry and Cell Biology, Department of Biofunction, Institute of Health Sciences, Federal University of Bahia (UFBA), Reitor Miguel Calmon Avenue, 1272-Canela, Salvador, BA 40231-300, Brazil; [email protected] 4 Laboratory of Research in Materia Medica, Department of Medicament, Faculty of Pharmacy, Federal University of Bahia (UFBA), Barão de Jeremoabo Street, 147-Ondina, Salvador, BA 40170-115, Brazil; [email protected] 5 Department of Organic Chemistry, Institute of Chemistry, Federal University of Bahia (UFBA), Barão de Jeremoabo Street, 147-Ondina, Salvador, BA 40170-115, Brazil; [email protected] * Correspondence: [email protected]; Tel.: +55-71-987-938-847 Received: 23 December 2017; Accepted: 11 January 2018; Published: 15 January 2018 Abstract: Background: A number of medicinal plants are traditionally used for metabolic disorders in Bahia state, Brazil. The aim of this study was to evaluate the estrogen receptor (ER) and thyroid receptor (TR) activation of crude extracts prepared from 20 plants. -

Development of Composition of Medicinal Cosmetic Cream of Anti
The Pharma Innovation Journal 2016; 5(1): 35-38 ISSN: 2277- 7695 TPI 2016; 5(1): 35-38 Development of composition of medicinal cosmetic © 2016 TPI cream of anti-inflammatory action www.thepharmajournal.com Received: 16-11-2015 Accepted: 17-12-2015 Horlachova VІ, Vyshnevskaya LІ Horlachova VІ National University of Abstract Pharmacy, Chemical technology of drugs, Kharkiv, Ukraine. Preliminary composition of the medicinal product has been determined. Тheoretically and experimentally, by pharmacological screening, the structure of medicinal cosmetic products in a form of Vyshnevskaya LІ cream being developed has been approved, in order to be used for dermatological diseases and skin National University of damages, namely: first degree burns, various skin inflammation and irritations, dermatitis. The Pharmacy, Chemical technology phytocomposition 2 has been choosed (fruit of wild carrot, flowers of Moroccan chamomile and Aloe of drugs, Kharkiv, Ukraine. leaves) which will effect anti-inflammatory, wound healing, burntreating, regenerating, hydrating, softening, analgesic and antioxidative activity. Keywords: phytocomposition, cream, anti-inflammatory action, medical herbal raw, medical cosmetic product. 1. Introduction Today, skin diseases are the common medical problems. Its state is one of the indicators of the total body health. Skin can perform its required functions in a good condition only. Therefore it is important to look carefully after it and take necessary measures to prevent the loss of elasticity and tone, and also premature wrinkles forming. It is known, that even minimal UV skin damage leads to negative consequences. UV-rays destroy cells of the upper layers of skin, damage blood capillaries, that causes to feeling of pain, heartburn and all signs of inflammation – sunburn [3, 6]. -

Investigation of Genus Rumex for Their Biologically Active Constituents
Nusrat Shafiq et al RJLBPCS 2017 www.rjlbpcs.com Life Science Informatics Publications Original Research Article DOI - 10.26479/2017.0206.11 INVESTIGATION OF GENUS RUMEX FOR THEIR BIOLOGICALLY ACTIVE CONSTITUENTS Nusrat Shafiq1*, Muhammad Saleem2, Sumaira Kousar1, Madiha Sahar3 and Syed Mahboob Hussain 4, Farhat Jabeen1 1. Department of Chemistry, Government College Women University-38000, Faisalabad, Pakistan 2. Department of Chemistry, The Islamia University of Bahawalpur-63100, Bahawalpur, Pakistan 3. Department of Chemistry, Government College University-38000, Faisalabad, Pakistan 4. Department of Chemistry University of Wah-47040, Wah Cantt., Pakistan ABSTRACT: The Potential of natural products to serve as effective and safe pharmaceutical agents have been increased due to the growing need of anti-inflammatory, anti-cancer and anti-biotic agents. Rural areas population of the Afro-Asian countries rely on the traditional medicinal systems to cure their ailments and largely based on the use of roots, leaves, fruits and flowers of different medicinal plant. A large number of sources of natural products, including plants, animals and minerals, have been found to be the basis of treatment of human diseases and have been explored to use by human for thousands of years. Pharmaceutical significance of the genus Rumex depends upon the occurrence of the chemical constituents which are found to have biological activities like anti-malarial, anti-inflammatory, anti-diarrheal, anti-viral activities and many other activities. Latest research was carried out on Rumex for its antioxidants, antibacterial, antiviral, antifungal, anti-mutagenic, anti-tumor and anti-suppressive activities The genus Rumex has attracted the attention of many investigators to isolate and analyze secondary metabolites due to its medicinal properties.