Plantago Media L.—Explored and Potential Applications of an Underutilized Plant
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

COMFORCLEANSE Item No
COMFORCLEANSE Item No. 320402 Size: 150 ct AUST L 193422 VEGAN PRODUCT SUMMARY & BACKGROUND ComforCleanse™ is an effective combination of herbs and essential oils that aids in the relief of constipation and helps reduce the occurrence of constipation. It effectively combines healthy fibre, minerals, herbs and essential oils to cleanse and balance. Conveniently packaged in capsule form, ComforCleanse also contains Cascara sagrada bark and psyllium. The colon is the final component of the digestive tract; it extracts water and salt from solid wastes before they are eliminated from the body. Although the bowel is comprised of the small and the large intestines, it is commonly used to describe the large intestines, which consist of the colon, the rectum and the anus. Therefore, a ‘bowel problem’ is any problem affecting these parts of the digestive tract. ComforCleanse was formulated to help cleanse and restore balance with the entire bowel in mind. KEY INGREDIENTS Berberis vulgaris, Burdock Root Powder, Chamomile Mentha piperita, pectin, Pimpinella anisum, Psyllium Oil German, Citrus reticulata, Copaiba Oil, Husk Powder, Rheum palmatum, Rosmarinus Echinacea purpurea, Ferula assa-foetida, Foeniculum officinalis, Zingiber officinale vulgare, Frangula purshiana, Garlic Clove Powder, FEATURES BENEFITS • Vegan-friendly formula • Decrease and relieve constipation • Contains Cascara sagrada (Frangula purshiana) • Can be used as a laxative bark traditionally used as a stimulant laxative • Helps reduce occurrence of constipation • Promotes bowel evacuation • Improves bowel waste elimination • Supports normal digestive and colon function WHO SHOULD TAKE COMFORCLEANSE? DIRECTIONS Those suffering from the uncomfortable effects of Adults take 2-3 capsules before breakfast and at constipation bedtime. Drink at least 2L of water throughout the day for best results. -

Central Composite Design for Optimizing the Biosynthesis Of
www.nature.com/scientificreports OPEN Central Composite Design for Optimizing the Biosynthesis of Silver Nanoparticles using Plantago major Extract and Investigating Antibacterial, Antifungal and Antioxidant Activity Ghazal Nikaeen1, Saeed Yousefnejad1 ✉ , Samane Rahmdel2, Fayezeh Samari3 & Saeideh Mahdavinia1 Central composite design (CCD) was applied to optimize the synthesis condition of silver nanoparticles (AgNPs) using the extract of Plantago major (P. major) seeds via a low cost and single-step process. The aqueous seed extract was applied as both reducing element and capping reagent for green production of AgNPs. Five empirical factors of synthesis including temperature (Temp), pH, volume of P. major extract (Vex), volume of AgNO3 solution (VAg) and synthesis time were used as independent variables of model and peak intensity of Surface Plasmon Resonance (SPR) originated from NPs as the dependent variable. The predicted optimal conditions was determined to be: Temp = 55 °C, pH = 9.9,Vex = 1.5 mL, VAg = 30 mL, time = 60 min. The characterization of the prepared AgNPs at these optimum conditions was conducted by Fourier transform infrared spectroscopy (FTIR), dynamic light scattering (DLS), transmission electron microscopy (TEM) and X-ray difraction (XRD) to determine the surface bio- functionalities. Bio-activity of these AgNPs against bacteria and fungi were evaluated based on its assay against Micrococcus luteus, Escherichia coli and Penicillium digitatum. Furthermore, antioxidant capacity of these NPs was checked using the ferric reducing antioxidant power (FRAP) assay. Nanotechnology is an important feld of modern research which has been the principal of various technologies and main innovations; and is expected to be the basis of many other outstanding innovations in future. -

Ornithocoprophilous Plants of Mount Desert Rock, a Remote Bird-Nesting Island in the Gulf of Maine, U.S.A
RHODORA, Vol. 111, No. 948, pp. 417–447, 2009 E Copyright 2009 by the New England Botanical Club ORNITHOCOPROPHILOUS PLANTS OF MOUNT DESERT ROCK, A REMOTE BIRD-NESTING ISLAND IN THE GULF OF MAINE, U.S.A. NISHANTA RAJAKARUNA Department of Biological Sciences, San Jose´ State University, One Washington Square, San Jose´, CA 95192-0100 e-mail: [email protected] NATHANIEL POPE AND JOSE PEREZ-OROZCO College of the Atlantic, 105 Eden Street, Bar Harbor, ME 04609 TANNER B. HARRIS University of Massachusetts, Fernald Hall, 270 Stockbridge Road, Amherst, MA 01003 ABSTRACT. Plants growing on seabird-nesting islands are uniquely adapted to deal with guano-derived soils high in N and P. Such ornithocoprophilous plants found in isolated, oceanic settings provide useful models for ecological and evolutionary investigations. The current study explored the plants foundon Mount Desert Rock (MDR), a small seabird-nesting, oceanic island 44 km south of Mount Desert Island (MDI), Hancock County, Maine, U.S.A. Twenty-seven species of vascular plants from ten families were recorded. Analyses of guano- derived soils from the rhizosphere of the three most abundant species from bird- 2 nesting sites of MDR showed significantly higher (P , 0.05) NO3 , available P, extractable Cd, Cu, Pb, and Zn, and significantly lower Mn compared to soils from the rhizosphere of conspecifics on non-bird nesting coastal bluffs from nearby MDI. Bio-available Pb was several-fold higher in guano soils than for background levels for Maine. Leaf tissue elemental analyses from conspecifics on and off guano soils showed significant differences with respect to N, Ca, K, Mg, Fe, Mn, Zn, and Pb, although trends were not always consistent. -

G.I. Fortify (Capsules) Introduced 2013
G.I. Fortify (capsules) Introduced 2013 What Is It? Recommendations G.I. Fortify (capsules) is designed to support G.I. health and bowel regularity as well Pure Encapsulations recommends 3-6 capsules daily, in divided doses, with a meal as promote a healthy G.I. environment, detoxification and colon cell function.* and 8-12 oz. water. Uses For G.I. Fortify (capsules) Are There Any Potential Side Effects Or Precautions? G.I. Motility: Psyllium, Plantago indica or blond psyllium, is a valued source of Not to be taken by pregnant or lactating women. Psyllium and flaxseed may cause soluble fiber. Soluble fiber increases stool volume when taken with appropriate gastrointestinal discomfort, including bloating, flatulence, addominal pain or amounts of water, supporting larger and softer stools for healthy bowel diarrhea. In rare cases, psyllium has been associated with headache, backache, movements. As the bulk moves through the intestine, it helps to collect and rhinitis, increased cough, and sinusitis. Psyllium should be consumed with eliminate other waste and toxins from the intestinal walls. This helps to minimize adequate water, as case reports indicate a potential for bowel obstruction when the amount of exposure of the gastrointestinal tract to toxins. Flaxseeds provide it is consumed without water. Rarely, individuals can have an allergic response a source of lignans, fatty acids, and both soluble and insoluble fibers, enhancing to psyllium, with symptoms including runny nose, sneezing, conjunctivitis, skin the gut health potential of this complex.* rash, itching, flushing, chest and throat tightness, congestion, hypotension or G.I. Integrity: l-Glutamine is the most abundant amino acid in the body. -

Site Synopsis
SITE SYNOPSIS Site Name: Inishtrahull SAC Site Code: 000154 This site is situated approximately 12.5 km north-east of Malin Head in Co. Donegal, and comprises the whole of the island of Inishtrahull, as well as a group of islands, the Tor Rocks, which lie approximately 2 km north-north-west of Inishtrahull and the intervening sea. The Tor Rocks, the most northerly point of land in Ireland, comprise six rocky pinnacles rising to approximately 20 m above the High Water Mark, with about eight sub-tidal rocks clustered about them. The island of Inishtrahull (34 ha) rises to 43 m at its western end and extends west-east for some 1.5 km. The geology of the site is of Lewisian gneiss, considered to be the oldest rock in Ireland, and having affinities with the rocks of southern Greenland and some of the Hebridean Islands. The soils found on Inishtrahull are either thin glacial tills or peaty podzols. The site is a Special Area of Conservation (SAC) selected for the following habitats and/or species listed on Annex I / II of the E.U. Habitats Directive (* = priority; numbers in brackets are Natura 2000 codes): [1230] Vegetated Sea Cliffs For most of its length the coastline of Inishtrahull consists of cliffs. These are not particularly high - the highest point of the island being only 43 m at the western end where the cliffs are best developed. The cliffs are generally not sheer, and are indented by deep clefts. Plant species associated with the cliffs include Thrift (Armeria maritima), Rock Sea-spurrey (Spergularia rupicola), Red Fescue (Festuca rubra), Common Scurvygrass (Cochlearia officinalis), Sea Campion (Silene vulgaris subsp. -

Plant Extracts Rich in Polyphenols: Antibacterial Agents and Natural Preservatives for Meat and Meat Products
Critical Reviews in Food Science and Nutrition ISSN: 1040-8398 (Print) 1549-7852 (Online) Journal homepage: https://www.tandfonline.com/loi/bfsn20 Plant extracts rich in polyphenols: antibacterial agents and natural preservatives for meat and meat products Magdalena Efenberger-Szmechtyk, Agnieszka Nowak & Agata Czyzowska To cite this article: Magdalena Efenberger-Szmechtyk, Agnieszka Nowak & Agata Czyzowska (2020): Plant extracts rich in polyphenols: antibacterial agents and natural preservatives for meat and meat products, Critical Reviews in Food Science and Nutrition, DOI: 10.1080/10408398.2020.1722060 To link to this article: https://doi.org/10.1080/10408398.2020.1722060 Published online: 11 Feb 2020. Submit your article to this journal Article views: 104 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=bfsn20 CRITICAL REVIEWS IN FOOD SCIENCE AND NUTRITION https://doi.org/10.1080/10408398.2020.1722060 REVIEW Plant extracts rich in polyphenols: antibacterial agents and natural preservatives for meat and meat products Magdalena Efenberger-Szmechtyk, Agnieszka Nowak, and Agata Czyzowska Institute of Fermentation Technology and Microbiology, Lodz University of Technology, Lodz, Poland ABSTRACT KEYWORDS Plant extracts contain large amounts of bioactive compounds, mainly polyphenols. Polyphenols Polyphenols; plant extracts; inhibit the growth of microorganisms, especially bacteria. Their mechanism of action is still not antibacterial activity; meat fully understood but may be related to their chemical structure. They can cause morphological changes in microorganisms, damage bacterial cell walls and influence biofilm formation. Polyphenols also influence protein biosynthesis, change metabolic processes in bacteria cells and inhibit ATP and DNA synthesis (suppressing DNA gyrase). -

Dcaeee2e425a50757de5fd8dedf
http://dx.doi.org/10.5935/0100-4042.20160063 Quim. Nova, Vol. 39, No. 4, 530-533, 2016 UNREMITTING PROBLEMS WITH CHLOROGENIC ACID NOMENCLATURE: A REVIEW Daniel Kremr, Tomáš Bajer, Petra Bajerová*, Silvie Surmová, and Karel Ventura University of Pardubice, Faculty of Chemical Technology, Department of Analytical Chemistry, Studentská 573, 532 10 Pardubice, Czech Republic Recebido em 08/12/2015; aceito em 12/01/2016; publicado na web em 12/04/2016 Assuntos Gerais This paper summarizes a problematic nomenclature of isomers belonging to chlorogenic acid family since its first occurrence until present. During decades, there have been a high number of articles dealing with the family. Unfortunately, researchers who want to get knowledge about this topic may be strongly confused after reading a few articles. Due to gradual discoveries and isolations of the individual isomers from plenty of matrices and because of the changing system of terminology after these discoveries, discrepancies among articles are common. The cause of this confusion is that the main compound of the family, 5-caffeoylquinic acid (also well- known as chlorogenic acid), was truly called as 3-caffeoylquinic acid before 1976, when new rules for nomenclature were published. Many researchers and also chemicals suppliers, however, keep using the “pre-IUPAC” nomenclature and wrongly call 3-caffeoylquinic acid as chlorogenic acid, the main substituent of the family. Despite there have been some works struggling with this issue, the problem is still appearing. Therefore, the present work was written. Keywords: chlorogenic acid; neochlorogenic acid; nomenclature; coffee. INTRODUCTION nomenclature is still very common, thus the present paper focuses on a possibility of making this problem clear, thus helping further As is well known nowadays, chlorogenic acids (CGAs) are natu- authors to overcome potential misunderstandings of the nomencla- rally occurring compounds found in all higher plants. -
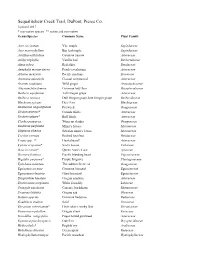
Sequalitchew Creek Trail Plant List
Sequalitchew Creek Trail, DuPont, Pierce Co. Updated 2017 * non-native species ** native and non-native Genus/Species Common Name Plant Family Acer circinatum Vine maple Sapindaceae Acer macrophyllum Big leaf maple Sapindaceae Achillea millefolium Common yarrow Asteraceae Achlys triphylla Vanilla leaf Berberidaceae Alnus rubra Red alder Betulaceae Anaphalis margaritacea Pearly everlasting Asteraceae Arbutus menziesii Pacific madrone Ericaceae Artemisia suksdorfii Coastal wormwood Asteraceae Asarum caudatum Wild ginger Aristolochiaceae Athyrium felix-femina Common lady fern Dryopteridaceae Berberis aquifolium Tall Oregon grape Asteraceae Berberis nervosa Dull Oregon grape, low Oregon grape Berberidaceae Blechnum spicant Deer fern Blechnaceae Chamerion angustifolium Fireweed Onagraceae Cirsium arvense* Canada thistle Asteraceae Cirsium vulgare* Bull thistle Asteraceae Clarkia purpurea Winecup clarkia Onagraceae Claytonia perfoliata Miner's lettuce Montiaceae Claytonia siberica Siberian miner's lettuce Montiaceae Corylus cornuta Beaked hazelnut Betulaceae Crepis spp. ?* Hawksbeard? Asteraceae Cytisus scoparius* Scot's broom Fabaceae Daucus carota* Queen Anne's Lace Apiaceae Dicentra formosa Pacific bleeding heart Papaveraceae Digitalis purpurea* Purple foxglove Plantaginaceae Epilobium minutum Threadstem fireweed Onagraceae Equisetum arvense Common horsetail Equisetaceae Equisetum telmateia Giant horsetail Equisetaceae Eriophyllum lanatum Oregon sunshine Asteraceae Erythronium oregonum White fawn lily Liliaceae Frangula purshiana Cascara, -

Checklist of the Vascular Plants of Redwood National Park
Humboldt State University Digital Commons @ Humboldt State University Botanical Studies Open Educational Resources and Data 9-17-2018 Checklist of the Vascular Plants of Redwood National Park James P. Smith Jr Humboldt State University, [email protected] Follow this and additional works at: https://digitalcommons.humboldt.edu/botany_jps Part of the Botany Commons Recommended Citation Smith, James P. Jr, "Checklist of the Vascular Plants of Redwood National Park" (2018). Botanical Studies. 85. https://digitalcommons.humboldt.edu/botany_jps/85 This Flora of Northwest California-Checklists of Local Sites is brought to you for free and open access by the Open Educational Resources and Data at Digital Commons @ Humboldt State University. It has been accepted for inclusion in Botanical Studies by an authorized administrator of Digital Commons @ Humboldt State University. For more information, please contact [email protected]. A CHECKLIST OF THE VASCULAR PLANTS OF THE REDWOOD NATIONAL & STATE PARKS James P. Smith, Jr. Professor Emeritus of Botany Department of Biological Sciences Humboldt State Univerity Arcata, California 14 September 2018 The Redwood National and State Parks are located in Del Norte and Humboldt counties in coastal northwestern California. The national park was F E R N S established in 1968. In 1994, a cooperative agreement with the California Department of Parks and Recreation added Del Norte Coast, Prairie Creek, Athyriaceae – Lady Fern Family and Jedediah Smith Redwoods state parks to form a single administrative Athyrium filix-femina var. cyclosporum • northwestern lady fern unit. Together they comprise about 133,000 acres (540 km2), including 37 miles of coast line. Almost half of the remaining old growth redwood forests Blechnaceae – Deer Fern Family are protected in these four parks. -

Towards Resolving Lamiales Relationships
Schäferhoff et al. BMC Evolutionary Biology 2010, 10:352 http://www.biomedcentral.com/1471-2148/10/352 RESEARCH ARTICLE Open Access Towards resolving Lamiales relationships: insights from rapidly evolving chloroplast sequences Bastian Schäferhoff1*, Andreas Fleischmann2, Eberhard Fischer3, Dirk C Albach4, Thomas Borsch5, Günther Heubl2, Kai F Müller1 Abstract Background: In the large angiosperm order Lamiales, a diverse array of highly specialized life strategies such as carnivory, parasitism, epiphytism, and desiccation tolerance occur, and some lineages possess drastically accelerated DNA substitutional rates or miniaturized genomes. However, understanding the evolution of these phenomena in the order, and clarifying borders of and relationships among lamialean families, has been hindered by largely unresolved trees in the past. Results: Our analysis of the rapidly evolving trnK/matK, trnL-F and rps16 chloroplast regions enabled us to infer more precise phylogenetic hypotheses for the Lamiales. Relationships among the nine first-branching families in the Lamiales tree are now resolved with very strong support. Subsequent to Plocospermataceae, a clade consisting of Carlemanniaceae plus Oleaceae branches, followed by Tetrachondraceae and a newly inferred clade composed of Gesneriaceae plus Calceolariaceae, which is also supported by morphological characters. Plantaginaceae (incl. Gratioleae) and Scrophulariaceae are well separated in the backbone grade; Lamiaceae and Verbenaceae appear in distant clades, while the recently described Linderniaceae are confirmed to be monophyletic and in an isolated position. Conclusions: Confidence about deep nodes of the Lamiales tree is an important step towards understanding the evolutionary diversification of a major clade of flowering plants. The degree of resolution obtained here now provides a first opportunity to discuss the evolution of morphological and biochemical traits in Lamiales. -

Pollen Morphology of Plantago Species Native to Poland and Their Taxonomic Implications
Vol. 73, No. 4: 315-325, 2004 ACTA SOCIETATIS BOTANICORUM POLONIAE 315 POLLEN MORPHOLOGY OF PLANTAGO SPECIES NATIVE TO POLAND AND THEIR TAXONOMIC IMPLICATIONS MA£GORZATA KLIMKO1, KRYSTYNA IDZIKOWSKA2, MARIOLA TRUCHAN3, ANNA KREFT3 1 Department of Botany, Agricultural University Wojska Polskiego 71C, 60-625 Poznañ, Poland e-mail: [email protected] 2 Laboratory of Electron Microscopy, Adam Mickiewicz University Grunwaldzka 6, 70-780 Poznañ, Poland 3 Department of Botany and Genetics, Institute of Biology and Environmental Protection, Pomeranian Pedagogical University Arciszewskiego 22B, 76-200 S³upsk, Poland (Received: March 29, 2004. Accepted: May 21, 2004) ABSTRACT Pollen grains of 9 species of the genus Plantago (Plantaginaceae), including 8 taxa native to Poland, were ob- served under a light microscope and a scanning electron microscope. Descriptions of grain sculpture are illustra- ted only SEM micrographs. The studied pollen grains were medium-sized or small, spherical or prolate spheroi- dal. Their sculpture was always verrucate with granulation. In the studied taxa, internal apertures had the form of pores. Their number ranged from (4)5-9(14). The pores were scattered on the surface of pollen grains. Identifica- tion features of individual taxa include: presence or absence of an annulus around each pore, annulus structure, ornamentation of the pollen grain and operculum, type of aperture membrane, number of internal pores, and pore diameter. We suggest that two new pollen grain types, characteristic of P. intermedia and P. arenaria, should be distinguished, and that P. alpina should be assigned to the P. coronopus type. KEY WORDS: Plantaginaceae, Plantago, pollen morphology, SEM. INTRODUCTION the sporophyte, whereas pollen size is determined both by sporophytic and gametophytic genotypes (Bedinger 1992; Pollen grains of the genus Plantago (Plantaginaceae) ha- McCormic 1993; Nepi et al.