A New Species of Bembidion Latrielle 1802 from the Ozarks, with a Review
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Beetle Fauna of Dominica, Lesser Antilles (Insecta: Coleoptera): Diversity and Distribution
INSECTA MUNDI, Vol. 20, No. 3-4, September-December, 2006 165 The beetle fauna of Dominica, Lesser Antilles (Insecta: Coleoptera): Diversity and distribution Stewart B. Peck Department of Biology, Carleton University, 1125 Colonel By Drive, Ottawa, Ontario K1S 5B6, Canada stewart_peck@carleton. ca Abstract. The beetle fauna of the island of Dominica is summarized. It is presently known to contain 269 genera, and 361 species (in 42 families), of which 347 are named at a species level. Of these, 62 species are endemic to the island. The other naturally occurring species number 262, and another 23 species are of such wide distribution that they have probably been accidentally introduced and distributed, at least in part, by human activities. Undoubtedly, the actual numbers of species on Dominica are many times higher than now reported. This highlights the poor level of knowledge of the beetles of Dominica and the Lesser Antilles in general. Of the species known to occur elsewhere, the largest numbers are shared with neighboring Guadeloupe (201), and then with South America (126), Puerto Rico (113), Cuba (107), and Mexico-Central America (108). The Antillean island chain probably represents the main avenue of natural overwater dispersal via intermediate stepping-stone islands. The distributional patterns of the species shared with Dominica and elsewhere in the Caribbean suggest stages in a dynamic taxon cycle of species origin, range expansion, distribution contraction, and re-speciation. Introduction windward (eastern) side (with an average of 250 mm of rain annually). Rainfall is heavy and varies season- The islands of the West Indies are increasingly ally, with the dry season from mid-January to mid- recognized as a hotspot for species biodiversity June and the rainy season from mid-June to mid- (Myers et al. -

The Purposes of This Paper Are to Clarify Generic Concepts in New
STUDIES OF THE SUBTRIBE TACHYINA (COLEOPTERA: CARABIDAE: BEMBIDIINI) SUPPLEMENT A: LECTOTYPE DESIGNATIONS FOR NEW WORLD SPECIES, TWO NEW GENERA, AND NOTES ON GENERIC CONCEPTS1 TERRY L. ERWIN National Museum of Natural History, Smithsonian Institution, Washington, D. C. 20560 ABSTRACT•The New World species-group names of the carabid subtribe Tachyina are arranged alphabetically by genus. Lectotype designation are made where necessary and species are assigned accordingly to their proper genus. Two new genera, Costitachys and Meotachys are described. Three species described in the genus Polyderis, testaceolimbata Motschulsky, glabrella Mots., and breviscula Mots., are reassigned to the genus Perigona of the Perigonini. A key is provided to Tachyina genera and notes on generic concepts are given. INTRODUCTION The purposes of this paper are to clarify generic concepts in New World Tachyina, designate lectotypes, list synonymies, provide a key to genera, and describe two new genera. Ail of this became possible after studying the World fauna to determine how New World groups relate to Old World groups. Much of this work has now been done and my series of revisions for the World Tachyina has begun to be issued (Erwin, 1973a, 1974). The work here has been strictly limited without giving reasons for many of the actions taken. Reasons will be provided in forthcoming revisions where space will allow full development of ideas from facts, and analyses of these facts. METHODS During 1971, I was able to study almost all primary type material for New World Tachyina as well as to study numerous Old World forms in the British Museum in London and in the Museum d'Histoire Naturelle in Paris. -

Coleoptera: Carabidae) by Thomas C
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Crossref TRECHOBLEMUS IN NORTH AMERICA, WITH A KEY TO NORTH AMERICAN GENERA OF TREX2HINAE (COLEOPTERA: CARABIDAE) BY THOMAS C. BARR, JR. The University of Kentucky, Lexington Trechoblemus Ganglbauer is a genus of trechine beetles (Tre- chinae: Trechini: Trechina) previously known only from Europe and Asia. It formed the type genus of Jeannel's "S6rie phyl6tique de Trechoblemus". and is generally regarded as closely related to cavernicolous trechines in Japan, the Carpathians and Transylvanian Alps of eastern Europe, and eastern United States (Barr, I969; Jeannel, I928, I962; U6no and Yoshida, I966). The large cave beetle genus Pseudanophthalmus Jeannel, with approximately 75 species in caves of ten eastern States, the monobasic genus Nea- phaenops Jeannel, from Kentucky caves, and the dibasic genus ]gelsonites Valentine, ]rorn Tennessee and Kentucky, are part of the Trechoblemus complex. The apparent restriction of Trechoblemus to Eurasia led previous investigators to conclude that, with respect to the richly diverse trechine fauna in caves of eastern United States, "there are no im- mediate, ancestral genera now present in North America" (Barr, 969, p. 83). Although there is at least one edaphobitic (obligate in soil) species of American Pseudanophthalmus known (P. sylvaticus Barr, I967), in the mountains of West Virginia, it has already lost eyes, wings, and pigment, and merely indicates that many of the "regressive" evolutionary changes in ancestral Pseudanohthal- mus may have taken place in the soil or deep humus before the beetles became restricted to caves. Most of the species of Pseuda- nophthalmus from eastern Europe (Barr, 964) are also eyeless edaphobites. -

Measuring Genome Sizes Using Read-Depth, K-Mers, and Flow Cytometry: Methodological Comparisons in Beetles (Coleoptera)
G3: Genes|Genomes|Genetics Early Online, published on June 29, 2020 as doi:10.1534/g3.120.401028 1 Measuring genome sizes using read-depth, k-mers, and flow 2 cytometry: methodological comparisons in beetles (Coleoptera) 3 James M. Pflug,*, Valerie Renee Holmes,† Crystal Burrus,† J. Spencer Johnston,† and David R. 4 Maddison* 5 6 *Department of Integrative Biology, Oregon State University, Corvallis, OR 97331, USA 7 †Department of Entomology, Texas A&M University, College Station, TX 77843, USA 8 1 Corresponding author: 3029 Cordley Hall, Department of Integrative Biology, Oregon State University, Corvallis, OR 97331 USA. E-mail: [email protected] © The Author(s) 2020. Published by the Genetics Society of America. 2 9 Abstract 10 Measuring genome size across different species can yield important insights into 11 evolution of the genome and allow for more informed decisions when designing next-generation 12 genomic sequencing projects. New techniques for estimating genome size using shallow 13 genomic sequence data have emerged which have the potential to augment our knowledge of 14 genome sizes, yet these methods have only been used in a limited number of empirical studies. In 15 this project, we compare estimation methods using next-generation sequencing (k-mer methods 16 and average read depth of single-copy genes) to measurements from flow cytometry, a standard 17 method for genome size measures, using ground beetles (Carabidae) and other members of the 18 beetle suborder Adephaga as our test system. We also present a new protocol for using read- 19 depth of single-copy genes to estimate genome size. -

Coleoptera: Carabidae, Trechinae)
Int. J. Speleo1.7 (1975), pp. 55-64. The Ecology of a Predaceous Troglobitic Beetle, Neaphaenops tellkampfii (Coleoptera: Carabidae, Trechinae) II. Adult Seasonality, Feeding and Recruitment. by Russell M. NORTON, Thomas C. KANE, Thomas L. POULSON INTRODUCTION This is the second of two papers dealing with the ecology of Neaphaenops. The procedure and a general introduction will be found in the first paper. RESULTS A. Adult Recruitment Recruitment of teneral adults in both Marion Avenue and Edwards Avenue shows a marked seasonality (Figure 7). Teneral adults emerge chiefly in late spring through early fall, although there is some emergence throughout the year. B. Adult Seasonality Census data for all adult Neaphaenops from the Marion Avenue and Edwards Ave- nue study areas are presented in Figures 5 and 6, respectively. Both areas show a summer through early fall population maximum coincident with the recruitment of teneral adults into the population, and a decrease through the rest of the year. Sex ratio data for both areas are presented in Table 3. Although the sex ratio for tenerals is 1: 1 (N)400), the sex ratio for fully sclerotized adults on sandy areas changes seasonally to a female majority (up to 2: I) just prior to the recruitment of sclerotized tenerals. C Adult Feeding The 46 feeding observations recorded for Neaphaenops to date are listed in Table 2. Of these, the ne plus ultra is a female in copula feeding on a Hadenoecus nymph. The two observations of Neaphaenops feeding on non cave-limited organisms were both made near entrances. About one third of the predated first instar Hadenoecus nymphs appeared to have been captured during emergence since they seemed to still be partially bound by the vitelline membrane. -
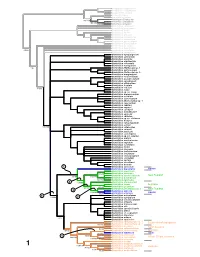
With Description of Bembidion Tahitiense, Sp. Nov. From
Asaphidion yukonense Asaphidion alaskanum Asaphidion curtum Lionepha erasa Lionepha osculans 99/100 Amerizus (Tiruka) sp. 100/100 Amerizus spectabilis 100/100 Amerizus wingatei Bembidion chalceum Bembidion properans Bembidion horni Bembidion hastii Bembidion planum Bembidion transversale Bembidion tetracolum Bembidion genei illigeri Bembidion geniculatum Bembidion cf. csikii Bembidion biguttatum 9 5/92 Bembidion wickhami Bembidion variegatum Bembidion ephippigerum Bembidion salinarium Bembidion assimile Bembidion nigrivestris Bembidion roosevelti Bembidion transparens 7 5/- Bembidion (Nothocys) sp. 1 Bembidion anthracinum Bembidion (Nothocys) sp. 2 Bembidion marginatum Bembidion fortestriatum Bembidion pseudocautum Bembidion canadianum Bembidion elizabethae Bembidion frontale 7 8/73 Bembidion siticum Bembidion lonae Bembidion sp. nr. lonae Bembidion melanopodum Bembidion scitulum Bembidion sexfoveatum Bembidion (Notholopha) sp. 1 Bembidion rugosellum Bembidion rawlinsi Bembidion rogersi Bembidion caoduroi Bembidion mandibulare Bembidion spinolai Bembidion chilense Bembidion sp. nr. chilense Bembidion hirtipes Bembidion rufoplagiatum Bembidion solieri 100/100 Bembidion cillenoides Bembidion calverti Bembidion posticale Bembidion convergens Bembidion sp. nr. ugartei Bembidion aratum Bembidion nubiculosum Bembidion rapidum Bembidion scintillans Bembidion flohri Bembidion idoneum Bembidion indistinctum Bembidion insulatum Bembidion obtusangulum Bembidion cordatum Bembidion varium Bembidion oberthueri Bembidion dorsale 5 Bembidion versutum -

Zootaxa,New Species of Anillinus Casey (Carabidae: Trechinae
Zootaxa 1542: 1–20 (2007) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2007 · Magnolia Press ISSN 1175-5334 (online edition) New species of Anillinus Casey (Carabidae: Trechinae: Bembidiini) from Great Smoky Mountains National Park, U.S.A. and phylogeography of the A. langdoni species group IGOR M. SOKOLOV1, YULIYA Y. SOKOLOVA2 & CHRISTOPHER E. CARLTON1 1Louisiana State Arthropod Museum, Department of Entomology, LSU Agricultural Center, Baton Rouge, Louisiana, 70803, USA. E-mail: [email protected]; [email protected] 2Laboratory for Insect Pathology, Department of Entomology, LSU Agricultural Center, Baton Rouge, Louisiana, 70803, USA. E-mail: [email protected] Abstract The Anillinus langdoni–species group is characterized and two new species are described, Anillinus cieglerae Sokolov and Carlton sp. nov. and A. pusillus Sokolov and Carlton sp. nov., both from Great Smoky Mountains National Park. The langdoni–group includes four species at present, three apparently endemic to the Great Smoky Mountains and adjacent mountains of western North Carolina/Tennessee, and a fourth from South Mountains of middle North Carolina. They are distinguished mainly using characters of the male genitalia and to a lesser extent, differences in shapes of female sper- mathecae. Phylogenetic analyses based on aedeagal morphology and COI gene sequences yielded conflicting results, with the later providing a phylogeny that was more parsimonious with expectations based on geographic distributions. Speciation within the group may derive from ecological constraints and altitudinal fluctuations of habitat corridors dur- ing past climate changes combined with the impact of local watersheds as fine scale isolating mechanisms. Key words: Coleoptera, Adephaga, Carabidae, Anillinus, South Appalachians, new species, taxonomy, identification key, COI gene sequences, phylogeography Introduction The genus Anillinus Casey is one of the most diverse genera of carabid beetles in the Southern Appalachian region of eastern United States. -

Your Name Here
RELATIONSHIPS BETWEEN DEAD WOOD AND ARTHROPODS IN THE SOUTHEASTERN UNITED STATES by MICHAEL DARRAGH ULYSHEN (Under the Direction of James L. Hanula) ABSTRACT The importance of dead wood to maintaining forest diversity is now widely recognized. However, the habitat associations and sensitivities of many species associated with dead wood remain unknown, making it difficult to develop conservation plans for managed forests. The purpose of this research, conducted on the upper coastal plain of South Carolina, was to better understand the relationships between dead wood and arthropods in the southeastern United States. In a comparison of forest types, more beetle species emerged from logs collected in upland pine-dominated stands than in bottomland hardwood forests. This difference was most pronounced for Quercus nigra L., a species of tree uncommon in upland forests. In a comparison of wood postures, more beetle species emerged from logs than from snags, but a number of species appear to be dependent on snags including several canopy specialists. In a study of saproxylic beetle succession, species richness peaked within the first year of death and declined steadily thereafter. However, a number of species appear to be dependent on highly decayed logs, underscoring the importance of protecting wood at all stages of decay. In a study comparing litter-dwelling arthropod abundance at different distances from dead wood, arthropods were more abundant near dead wood than away from it. In another study, ground- dwelling arthropods and saproxylic beetles were little affected by large-scale manipulations of dead wood in upland pine-dominated forests, possibly due to the suitability of the forests surrounding the plots. -

Appendix O19749
Oikos o19749 Gerisch, M., Agostinelli, V., Henle, K. and Dziock, F. 2011. More species, but all do the same: contrasting effects of flood disturbance on ground beetle functional and species diversity. – Oikos 121: 508–515. Appendix A1 Tabelle1 Table A1. Full species list representing the standardized number of individuals per species for the study sites Steckby, Woerlitz, and Sandau. Density expresses the proportion of species standardized abundances to total abundance. Macropterous = winged, brachypterous = wingless, dimorphic = both forms can appear with a species. Body size is the average of maximum and minimum values found in the literature (for references see below). Wing Reproduction Body size Species names Steckby Woerlitz Sandau Density Morphology Season In mm Acupalpus dubius 0.032 0 0.016 0 macropterous spring 2.6 Acupalpus exiguus 1.838 1.019 0.71 0.005 macropterous spring 2.7 Acupalpus parvulus 0.081 0.038 0.032 0 macropterous spring 3.6 Agonum dolens 0.032 0.038 0.081 0 dimorph spring 8.8 Agonum duftschmidi 14.966 2.755 0.016 0.025 macropterous spring 8.2 Agonum emarginatum 116.659 4.472 25.194 0.208 macropterous spring 7.2 Agonum fuliginosum 0.097 0.038 0 0 dimorph spring 6.7 Agonum lugens 0.177 0 0.081 0 macropterous spring 9 Agonum marginatum 0.371 0.075 0.113 0.001 macropterous spring 9.2 Agonum micans 19.502 4.208 23.71 0.067 macropterous spring 6.6 Agonum muelleri 0 0.019 0 0 macropterous spring 8.2 Agonum piceum 0.468 0 0.016 0.001 macropterous spring 6.4 Agonum sexpunctatum 0.032 0 0.016 0 macropterous spring 8.2 Agonum -

Holocene Palaeoenvironmental Reconstruction Based on Fossil Beetle Faunas from the Altai-Xinjiang Region, China
Holocene palaeoenvironmental reconstruction based on fossil beetle faunas from the Altai-Xinjiang region, China Thesis submitted for the degree of Doctor of Philosophy at the University of London By Tianshu Zhang February 2018 Department of Geography, Royal Holloway, University of London Declaration of Authorship I Tianshu Zhang hereby declare that this thesis and the work presented in it is entirely my own. Where I have consulted the work of others, this is always clearly stated. Signed: Date: 25/02/2018 1 Abstract This project presents the results of the analysis of fossil beetle assemblages extracted from 71 samples from two peat profiles from the Halashazi Wetland in the southern Altai region of northwest China. The fossil assemblages allowed the reconstruction of local environments of the early (10,424 to 9500 cal. yr BP) and middle Holocene (6374 to 4378 cal. yr BP). In total, 54 Coleoptera taxa representing 44 genera and 14 families have been found, and 37 species have been identified, including a new species, Helophorus sinoglacialis. The majority of the fossil beetle species identified are today part of the Siberian fauna, and indicate cold steppe or tundra ecosystems. Based on the biogeographic affinities of the fossil faunas, it appears that the Altai Mountains served as dispersal corridor for cold-adapted (northern) beetle species during the Holocene. Quantified temperature estimates were made using the Mutual Climate Range (MCR) method. In addition, indicator beetle species (cold adapted species and bark beetles) have helped to identify both cold and warm intervals, and moisture conditions have been estimated on the basis of water associated species. -

A New Species of Bembidion LATREILLE from Nemrut Dağ
©Wiener Coleopterologenverein (WCV), download unter www.biologiezentrum.at Koleopterologische Rundschau 76 7–13 Wien, Juli 2006 A new species of Bembidion LATREILLE from Nemrut Da÷, Turkey (Coleoptera: Carabidae) L. TOLEDANO &K.RÉBL Abstract Bembidion (Ocydromus) nemrutdagi sp.n. from the Nemrut Da÷ (Turkey, AdÕyaman Prov.) is described. Its nearest relative, B. cordicolle DUVAL, 1851, is transferred to the subgen. Ocydromus CLAIRVILLE, 1806 s.l. (sensu KRYZHANOVSKIJ et al. 1995). The systematic relationships of some species groups are discussed. Key words: Coleoptera, Carabidae, Bembidiina, Bembidion, Ocydromus, Turkey, taxonomy. Introduction While studying Turkish Bembidion LATREILLE, 1802 we detected two specimens extremely similar to B. cordicolle DUVAL, 1851, from which they could be distinguished by the number of elytral pores. After comparison with the other species occurring in the area and after a thorough literature study we concluded that these specimens represent an undescribed species. Material and methods This paper is based on the study of 60 specimens belonging to the species dealt with herein and several hundreds of other specimens belonging to the subgen. Ocydromus CLAIRVILLE, 1806 sensu KRYZHANOVSKIJ et al. (1995). Collection Acronyms: CF Coll. Facchini, Piacenza CM Coll. Moret, Madrid CN Coll. Neri, S. Lorenzo in Noceto, Forlì CR Coll. Rébl, Nové Strašecí CT Coll. Toledano, Verona NMW Coll. Naturhistorisches Museum Wien The measurements, made with a Leica MZ12 stereobinocular microscope at 25 x (body) and 100 x (median lobes of aedeagi), are expressed in the text by the following abbreviations: el/ew elytral length / elytral width ratio ew/pw elytral width / pronotum width ratio pw/hw pronotum width / head width ratio pw/pl pronotum width / pronotum length ratio The body length has been measured from the front margin of the clypeus to the apex of the elytra, and the antennal length from the base of the antennomere 1 to the apex of antennomere 11. -

Carabidae Recording Card A4
Locality Grey cells for GPS RA77 COLEOPTERA: Carabidae (6453) Vice-county Grid reference users Recording Form Recorder Determiner Compiler Source (tick one) Date(s) from: Habitat (optional) Altitude Field to: (metres) Museum* *Source details No. No. No. Literature* OMOPHRONINAE 21309 Dyschirius politus 22335 Bembidion nigricorne 22717 Pterostichus niger 23716 Amara familiaris 24603 Stenolophus teutonus 25805 Dromius melanocephalus 20201 Omophron limbatum 21310 Dyschirius salinus 22336 Bembidion nigropiceum 22724 Pterostichus nigrita agg. 23717 Amara fulva 24501 Bradycellus caucasicus 25806 Dromius meridionalis CARABINAE 21311 Dyschirius thoracicus 22338 Bembidion normannum 22718 Pterostichus nigrita s.s. 23718 Amara fusca 24502 Bradycellus csikii 25807 Dromius notatus 20501 Calosoma inquisitor 21401 Clivina collaris 22339 Bembidion obliquum 22723 Pterostichus rhaeticus 23719 Amara infima 24503 Bradycellus distinctus 25808 Dromius quadrimaculatus 20502 Calosoma sycophanta 21402 Clivina fossor 22340 Bembidion obtusum 22719 Pterostichus oblongopunctatus 23720 Amara lucida 24504 Bradycellus harpalinus 25810 Dromius quadrisignatus 20401 Carabus arvensis BROSCINAE 22341 Bembidion octomaculatum 22703 Pterostichus quadrifoveolatus 23721 Amara lunicollis 24505 Bradycellus ruficollis 25811 Dromius sigma 20402 Carabus auratus 21501 Broscus cephalotes 22342 Bembidion pallidipenne 22720 Pterostichus strenuus 23722 Amara montivaga 24506 Bradycellus sharpi 25809 Dromius spilotus 20404 Carabus clathratus 21601 Miscodera arctica 22343 Bembidion prasinum