Ground Beetles in AB
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
A New Species of Bembidion Latrielle 1802 from the Ozarks, with a Review
A peer-reviewed open-access journal ZooKeys 147: 261–275 (2011)A new species of Bembidion Latrielle 1802 from the Ozarks... 261 doi: 10.3897/zookeys.147.1872 RESEARCH ARTICLE www.zookeys.org Launched to accelerate biodiversity research A new species of Bembidion Latrielle 1802 from the Ozarks, with a review of the North American species of subgenus Trichoplataphus Netolitzky 1914 (Coleoptera, Carabidae, Bembidiini) Drew A. Hildebrandt1,†, David R. Maddison2,‡ 1 710 Laney Road, Clinton, MS 39056 USA 2 Department of Zoology, Oregon State University, Corvallis, OR 97331, USA † urn:lsid:zoobank.org:author:038776CA-F70A-4744-96D6-B9B43FB56BB4 ‡ urn:lsid:zoobank.org:author:075A5E9B-5581-457D-8D2F-0B5834CDE04D Corresponding author: David R. Maddison ([email protected]) Academic editor: T. Erwin | Received 31 July 2011 | Accepted 25 August 2011 | Published 16 November 2011 urn:lsid:zoobank.org:pub:52038529-10EA-41A8-BE4F-6B495B610900 Citation: Hildebrandt DA, Maddison DR (2011) A new species of Bembidion Latrielle 1802 from the Ozarks, with a review of the North American species of subgenus Trichoplataphus Netolitzky 1914 (Coleoptera, Carabidae, Bembidiini). In: Erwin T (Ed) Proceedings of a symposium honoring the careers of Ross and Joyce Bell and their contributions to scientific work. Burlington, Vermont, 12–15 June 2010. ZooKeys 147: 261–275. doi: 10.3897/zookeys.147.1872 Abstract A new species of Bembidion (Trichoplataphus Netolitzky) from the Ozark Plateau of Missouri and Arkan- sas is described (Bembidion ozarkense Maddison and Hildebrandt). It is distinguishable from the closely related species, B. rolandi Fall, by characteristics of the male genitalia, and sequences of the genes cyto- chrome oxidase I and 28S ribosomal DNA. -

Coleoptera: Carabidae) Assemblages in a North American Sub-Boreal Forest
Forest Ecology and Management 256 (2008) 1104–1123 Contents lists available at ScienceDirect Forest Ecology and Management journal homepage: www.elsevier.com/locate/foreco Catastrophic windstorm and fuel-reduction treatments alter ground beetle (Coleoptera: Carabidae) assemblages in a North American sub-boreal forest Kamal J.K. Gandhi a,b,1, Daniel W. Gilmore b,2, Steven A. Katovich c, William J. Mattson d, John C. Zasada e,3, Steven J. Seybold a,b,* a Department of Entomology, 219 Hodson Hall, 1980 Folwell Avenue, University of Minnesota, St. Paul, MN 55108, USA b Department of Forest Resources, 115 Green Hall, University of Minnesota, St. Paul, MN 55108, USA c USDA Forest Service, State and Private Forestry, 1992 Folwell Avenue, St. Paul, MN 55108, USA d USDA Forest Service, Northern Research Station, Forestry Sciences Laboratory, 5985 Hwy K, Rhinelander, WI 54501, USA e USDA Forest Service, Northern Research Station, 1831 Hwy 169E, Grand Rapids, MN 55744, USA ARTICLE INFO ABSTRACT Article history: We studied the short-term effects of a catastrophic windstorm and subsequent salvage-logging and Received 9 September 2007 prescribed-burning fuel-reduction treatments on ground beetle (Coleoptera: Carabidae) assemblages in a Received in revised form 8 June 2008 sub-borealforestinnortheasternMinnesota,USA. During2000–2003, 29,873groundbeetlesrepresentedby Accepted 9 June 2008 71 species were caught in unbaited and baited pitfall traps in aspen/birch/conifer (ABC) and jack pine (JP) cover types. At the family level, both land-area treatment and cover type had significant effects on ground Keywords: beetle trap catches, but there were no effects of pinenes and ethanol as baits. -

Arthropod Diversity and Conservation in Old-Growth Northwest Forests'
AMER. ZOOL., 33:578-587 (1993) Arthropod Diversity and Conservation in Old-Growth mon et al., 1990; Hz Northwest Forests complex litter layer 1973; Lattin, 1990; JOHN D. LATTIN and other features Systematic Entomology Laboratory, Department of Entomology, Oregon State University, tural diversity of th Corvallis, Oregon 97331-2907 is reflected by the 14 found there (Lawtt SYNOPSIS. Old-growth forests of the Pacific Northwest extend along the 1990; Parsons et a. e coastal region from southern Alaska to northern California and are com- While these old posed largely of conifer rather than hardwood tree species. Many of these ity over time and trees achieve great age (500-1,000 yr). Natural succession that follows product of sever: forest stand destruction normally takes over 100 years to reach the young through successioi mature forest stage. This succession may continue on into old-growth for (Lattin, 1990). Fire centuries. The changing structural complexity of the forest over time, and diseases, are combined with the many different plant species that characterize succes- bances. The prolot sion, results in an array of arthropod habitats. It is estimated that 6,000 a continually char arthropod species may be found in such forests—over 3,400 different ments and habitat species are known from a single 6,400 ha site in Oregon. Our knowledge (Southwood, 1977 of these species is still rudimentary and much additional work is needed Lawton, 1983). throughout this vast region. Many of these species play critical roles in arthropods have lx the dynamics of forest ecosystems. They are important in nutrient cycling, old-growth site, tt as herbivores, as natural predators and parasites of other arthropod spe- mental Forest (HJ cies. -

Invertebrate Distribution and Diversity Assessment at the U. S. Army Pinon Canyon Maneuver Site a Report to the U
Invertebrate Distribution and Diversity Assessment at the U. S. Army Pinon Canyon Maneuver Site A report to the U. S. Army and U. S. Fish and Wildlife Service G. J. Michels, Jr., J. L. Newton, H. L. Lindon, and J. A. Brazille Texas AgriLife Research 2301 Experiment Station Road Bushland, TX 79012 2008 Report Introductory Notes The invertebrate survey in 2008 presented an interesting challenge. Extremely dry conditions prevailed throughout most of the adult activity period for the invertebrates and grass fires occurred several times throughout the summer. By visual assessment, plant resources were scarce compared to last year, with few green plants and almost no flowering plants. Eight habitats and nine sites continued to be sampled in 2008. The Ponderosa pine/ yellow indiangrass site was removed from the study after the low numbers of species and individuals collected there in 2007. All other sites from the 2007 survey were included in the 2008 survey. We also discontinued the collection of Coccinellidae in the 2008 survey, as only 98 individuals from four species were collected in 2007. Pitfall and malaise trapping were continued in the same way as the 2007 survey. Sweep net sampling was discontinued to allow time for Asilidae and Orthoptera timed surveys consisting of direct collection of individuals with a net. These surveys were conducted in the same way as the time constrained butterfly (Papilionidea and Hesperoidea) surveys, with 15-minute intervals for each taxanomic group. This was sucessful when individuals were present, but the dry summer made it difficult to assess the utility of these techniques because of overall low abundance of insects. -

Tropical Insect Chemical Ecology - Edi A
TROPICAL BIOLOGY AND CONSERVATION MANAGEMENT – Vol.VII - Tropical Insect Chemical Ecology - Edi A. Malo TROPICAL INSECT CHEMICAL ECOLOGY Edi A. Malo Departamento de Entomología Tropical, El Colegio de la Frontera Sur, Carretera Antiguo Aeropuerto Km. 2.5, Tapachula, Chiapas, C.P. 30700. México. Keywords: Insects, Semiochemicals, Pheromones, Kairomones, Monitoring, Mass Trapping, Mating Disrupting. Contents 1. Introduction 2. Semiochemicals 2.1. Use of Semiochemicals 3. Pheromones 3.1. Lepidoptera Pheromones 3.2. Coleoptera Pheromones 3.3. Diptera Pheromones 3.4. Pheromones of Insects of Medical Importance 4. Kairomones 4.1. Coleoptera Kairomones 4.2. Diptera Kairomones 5. Synthesis 6. Concluding Remarks Acknowledgments Glossary Bibliography Biographical Sketch Summary In this chapter we describe the current state of tropical insect chemical ecology in Latin America with the aim of stimulating the use of this important tool for future generations of technicians and professionals workers in insect pest management. Sex pheromones of tropical insectsUNESCO that have been identified to– date EOLSS are mainly used for detection and population monitoring. Another strategy termed mating disruption, has been used in the control of the tomato pinworm, Keiferia lycopersicella, and the Guatemalan potato moth, Tecia solanivora. Research into other semiochemicals such as kairomones in tropical insects SAMPLErevealed evidence of their presence CHAPTERS in coleopterans. However, additional studies are necessary in order to confirm these laboratory results. In fruit flies, the isolation of potential attractants (kairomone) from Spondias mombin for Anastrepha obliqua was reported recently. The use of semiochemicals to control insect pests is advantageous in that it is safe for humans and the environment. The extensive use of these kinds of technologies could be very important in reducing the use of pesticides with the consequent reduction in the level of contamination caused by these products around the world. -

Scottish Beetles Introduction to Ground Beetles (Carabidae) There Are Almost 400 Species of Ground Beetles in the UK
Scottish Beetles Introduction to Ground Beetles (Carabidae) There are almost 400 species of ground beetles in the UK. This guide is an introduction to 17 species found in this family. It is intended to be used in combination with the beetle anatomy guide and survey and recording guides. This family has species in a wide variety of sizes ranging from 2 to 30 mm. There are 5 tarsal segments on each foot. These beetles generally have long antennae and long legs, and often, but not always elongated oval body shapes. Beetles in this family range in colour from black, through many metallic colours, to bright green. Many of the species of beetles found in Scotland need careful examination with a microscope to identify them. This guide is designed to introduce some of the ground beetles you may find and give some key identification features for each. Violet ground beetle (Carabus violaceus) 20-30mm A large black beetle with metallic blue or purple edging, this beetle can often be confused with Carabus problematicus, however, it has smoother, more finely granulated elytra, whereas C. problematicus has elytra that are more rounded, more strongly sculptured and less convex. The Violet ground beetle pronotum is more convex. Where to look - Found in woodlands and moorlands. Occasionally seen on paths from April to September. Found everywhere in Scotland except the Western Isles, Mull and the Shetlands. BY 2.0 CC Shcmidt © Udo Ridged violet ground beetle (Carabus problematicus) 20-28mm This is a large black beetle with blue or violet tinted edges to the thorax and elytra. -

Coleoptera: Carabidae) Diversity
VEGETATIVE COMMUNITIES AS INDICATORS OF GROUND BEETLE (COLEOPTERA: CARABIDAE) DIVERSITY BY ALAN D. YANAHAN THESIS Submitted in partial fulfillment of the requirements for the degree of Master of Science in Entomology in the Graduate College of the University of Illinois at Urbana-Champaign, 2013 Urbana, Illinois Master’s Committee: Dr. Steven J. Taylor, Chair, Director of Research Adjunct Assistant Professor Sam W. Heads Associate Professor Andrew V. Suarez ABSTRACT Formally assessing biodiversity can be a daunting if not impossible task. Subsequently, specific taxa are often chosen as indicators of patterns of diversity as a whole. Mapping the locations of indicator taxa can inform conservation planning by identifying land units for management strategies. For this approach to be successful, though, land units must be effective spatial representations of the species assemblages present on the landscape. In this study, I determined whether land units classified by vegetative communities predicted the community structure of a diverse group of invertebrates—the ground beetles (Coleoptera: Carabidae). Specifically, that (1) land units of the same classification contained similar carabid species assemblages and that (2) differences in species structure were correlated with variation in land unit characteristics, including canopy and ground cover, vegetation structure, tree density, leaf litter depth, and soil moisture. The study site, the Braidwood Dunes and Savanna Nature Preserve in Will County, Illinois is a mosaic of differing land units. Beetles were sampled continuously via pitfall trapping across an entire active season from 2011–2012. Land unit characteristics were measured in July 2012. Nonmetric multidimensional scaling (NMDS) ordinated the land units by their carabid assemblages into five ecologically meaningful clusters: disturbed, marsh, prairie, restoration, and savanna. -

2009 Pinon Canyon Invertebrate Survey Report
"- - 70.096 60.096 50.096 40.096 30.096 20.096 10.096 0.0% Fig. 1 Most abundant Apiformes species calculated as a proportion of the total abundance of Apiformes in the collection period. Pinon Canyon Maneuver Site, 2008. 04% 1 j 0.391> 0.2% 0.1% 0.0% Fig. 2 Least abundant Apiformes species calculated as a proportion of the total abundance of Apiformes in the collection period. Pinon Canyon Maneuver Site, 2008.7 Fig. 3 Most abundant Carabidae species calculated as a proportion of the total abundance of Carabidae in the collection period. Pinon Canyon Maneuver Site, 2008. Fig. 4 Least abundant Carabidae species calculated as a proportion of the total abundance of Carabidae in the collection period. Pinon Canyon Maneuver Site, 2008. Fig. 5 Asilidae species abundance calculated as a proportion of the total abundace of Asilidae in the collection period. Pinon Canyon Maneuver Site, 2008. 30.0% 25.0% 20.0% 15.0% 10.0% 5.0% 0.0% Fig. 6 Butterfly species abundance calculated as a proportion of the total abundance of butterflies in the collection period. Pinon Canyon Maneuver Site, 2008. Fig. 7 Most abundant Orthoptera species calculated as a proportion of the total abundance of Orthoptera in the collection period. Pinon Canyon Maneuver Site, 2008. Fig. 8 Moderately abundant Orthoptera species calculated as a proportion of the total abundance of Orthoptera in the collection period. Pinon Canyon Maneuver Site, 2008. Fig. 9 Least abundant Orthoptera species calculated as a proportion of the total abundance of Orthoptera in the collection period. -

ARTHROPODA Subphylum Hexapoda Protura, Springtails, Diplura, and Insects
NINE Phylum ARTHROPODA SUBPHYLUM HEXAPODA Protura, springtails, Diplura, and insects ROD P. MACFARLANE, PETER A. MADDISON, IAN G. ANDREW, JOCELYN A. BERRY, PETER M. JOHNS, ROBERT J. B. HOARE, MARIE-CLAUDE LARIVIÈRE, PENELOPE GREENSLADE, ROSA C. HENDERSON, COURTenaY N. SMITHERS, RicarDO L. PALMA, JOHN B. WARD, ROBERT L. C. PILGRIM, DaVID R. TOWNS, IAN McLELLAN, DAVID A. J. TEULON, TERRY R. HITCHINGS, VICTOR F. EASTOP, NICHOLAS A. MARTIN, MURRAY J. FLETCHER, MARLON A. W. STUFKENS, PAMELA J. DALE, Daniel BURCKHARDT, THOMAS R. BUCKLEY, STEVEN A. TREWICK defining feature of the Hexapoda, as the name suggests, is six legs. Also, the body comprises a head, thorax, and abdomen. The number A of abdominal segments varies, however; there are only six in the Collembola (springtails), 9–12 in the Protura, and 10 in the Diplura, whereas in all other hexapods there are strictly 11. Insects are now regarded as comprising only those hexapods with 11 abdominal segments. Whereas crustaceans are the dominant group of arthropods in the sea, hexapods prevail on land, in numbers and biomass. Altogether, the Hexapoda constitutes the most diverse group of animals – the estimated number of described species worldwide is just over 900,000, with the beetles (order Coleoptera) comprising more than a third of these. Today, the Hexapoda is considered to contain four classes – the Insecta, and the Protura, Collembola, and Diplura. The latter three classes were formerly allied with the insect orders Archaeognatha (jumping bristletails) and Thysanura (silverfish) as the insect subclass Apterygota (‘wingless’). The Apterygota is now regarded as an artificial assemblage (Bitsch & Bitsch 2000). -
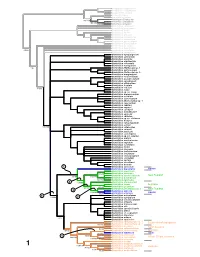
With Description of Bembidion Tahitiense, Sp. Nov. From
Asaphidion yukonense Asaphidion alaskanum Asaphidion curtum Lionepha erasa Lionepha osculans 99/100 Amerizus (Tiruka) sp. 100/100 Amerizus spectabilis 100/100 Amerizus wingatei Bembidion chalceum Bembidion properans Bembidion horni Bembidion hastii Bembidion planum Bembidion transversale Bembidion tetracolum Bembidion genei illigeri Bembidion geniculatum Bembidion cf. csikii Bembidion biguttatum 9 5/92 Bembidion wickhami Bembidion variegatum Bembidion ephippigerum Bembidion salinarium Bembidion assimile Bembidion nigrivestris Bembidion roosevelti Bembidion transparens 7 5/- Bembidion (Nothocys) sp. 1 Bembidion anthracinum Bembidion (Nothocys) sp. 2 Bembidion marginatum Bembidion fortestriatum Bembidion pseudocautum Bembidion canadianum Bembidion elizabethae Bembidion frontale 7 8/73 Bembidion siticum Bembidion lonae Bembidion sp. nr. lonae Bembidion melanopodum Bembidion scitulum Bembidion sexfoveatum Bembidion (Notholopha) sp. 1 Bembidion rugosellum Bembidion rawlinsi Bembidion rogersi Bembidion caoduroi Bembidion mandibulare Bembidion spinolai Bembidion chilense Bembidion sp. nr. chilense Bembidion hirtipes Bembidion rufoplagiatum Bembidion solieri 100/100 Bembidion cillenoides Bembidion calverti Bembidion posticale Bembidion convergens Bembidion sp. nr. ugartei Bembidion aratum Bembidion nubiculosum Bembidion rapidum Bembidion scintillans Bembidion flohri Bembidion idoneum Bembidion indistinctum Bembidion insulatum Bembidion obtusangulum Bembidion cordatum Bembidion varium Bembidion oberthueri Bembidion dorsale 5 Bembidion versutum -

Your Name Here
RELATIONSHIPS BETWEEN DEAD WOOD AND ARTHROPODS IN THE SOUTHEASTERN UNITED STATES by MICHAEL DARRAGH ULYSHEN (Under the Direction of James L. Hanula) ABSTRACT The importance of dead wood to maintaining forest diversity is now widely recognized. However, the habitat associations and sensitivities of many species associated with dead wood remain unknown, making it difficult to develop conservation plans for managed forests. The purpose of this research, conducted on the upper coastal plain of South Carolina, was to better understand the relationships between dead wood and arthropods in the southeastern United States. In a comparison of forest types, more beetle species emerged from logs collected in upland pine-dominated stands than in bottomland hardwood forests. This difference was most pronounced for Quercus nigra L., a species of tree uncommon in upland forests. In a comparison of wood postures, more beetle species emerged from logs than from snags, but a number of species appear to be dependent on snags including several canopy specialists. In a study of saproxylic beetle succession, species richness peaked within the first year of death and declined steadily thereafter. However, a number of species appear to be dependent on highly decayed logs, underscoring the importance of protecting wood at all stages of decay. In a study comparing litter-dwelling arthropod abundance at different distances from dead wood, arthropods were more abundant near dead wood than away from it. In another study, ground- dwelling arthropods and saproxylic beetles were little affected by large-scale manipulations of dead wood in upland pine-dominated forests, possibly due to the suitability of the forests surrounding the plots. -

Appendix O19749
Oikos o19749 Gerisch, M., Agostinelli, V., Henle, K. and Dziock, F. 2011. More species, but all do the same: contrasting effects of flood disturbance on ground beetle functional and species diversity. – Oikos 121: 508–515. Appendix A1 Tabelle1 Table A1. Full species list representing the standardized number of individuals per species for the study sites Steckby, Woerlitz, and Sandau. Density expresses the proportion of species standardized abundances to total abundance. Macropterous = winged, brachypterous = wingless, dimorphic = both forms can appear with a species. Body size is the average of maximum and minimum values found in the literature (for references see below). Wing Reproduction Body size Species names Steckby Woerlitz Sandau Density Morphology Season In mm Acupalpus dubius 0.032 0 0.016 0 macropterous spring 2.6 Acupalpus exiguus 1.838 1.019 0.71 0.005 macropterous spring 2.7 Acupalpus parvulus 0.081 0.038 0.032 0 macropterous spring 3.6 Agonum dolens 0.032 0.038 0.081 0 dimorph spring 8.8 Agonum duftschmidi 14.966 2.755 0.016 0.025 macropterous spring 8.2 Agonum emarginatum 116.659 4.472 25.194 0.208 macropterous spring 7.2 Agonum fuliginosum 0.097 0.038 0 0 dimorph spring 6.7 Agonum lugens 0.177 0 0.081 0 macropterous spring 9 Agonum marginatum 0.371 0.075 0.113 0.001 macropterous spring 9.2 Agonum micans 19.502 4.208 23.71 0.067 macropterous spring 6.6 Agonum muelleri 0 0.019 0 0 macropterous spring 8.2 Agonum piceum 0.468 0 0.016 0.001 macropterous spring 6.4 Agonum sexpunctatum 0.032 0 0.016 0 macropterous spring 8.2 Agonum