Reassessing the Scottish Mesolithic-Neolithic Transition: Questions of Diet and Chronology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Paleolithic and Mesolithic Occupation of the Isle of Jura, Argyll
John MERCER, Edinburgh THE PALAEOLITHIC AND MESOLITHIC OCCUPATION OF THE ISLE OF JURA, ARGYLL, SCOTLAND The occupation sequence about to be described has been built up from a dozen sites concentrated in N-Jura (Mercer, 1968-79).It is based on local land-sea relationships, site stratification, pollen analysis, drifted-pumice dating and radiocarbon assay.The paper 1 will begin with a discussion of the inter-linked shorelines and climate, then give an impression of the main sites and, finally, describe and compare the stone implement typology. Late Glacial habitat 2017 Jura is a vast island (fig.1) some 80 km (50 m) long.It rises to about 780 m (2500ft) Biblioteca, in the south and 470 m (1500 ft)in the north. Several recent papers have shown that W-Scotland was suitable for human habita ULPGC. tion from 11,000 or 10,500 BC. Kirk and Godwin (1963) described an organic level por at Loch Drama (Ross and Cromarty) which, with a C14 date of 12,810 ± 155 be (Q-457), had not since been overlaid by ice, although in a through valley.Kirk com realizada mented: "In view of its location on the exposed, north-west Atlantic rim of Scotland one would except ...an onset of milder oceanic conditions at an earlier date than localities in the English Lowlands or the North European Plain." He concluded his Digitalización contribution: " ... it would appear that in Northern Scotland the process of degla ciation was not unlike that established for Scandinavia, namely an early and rapid autores. los melt of the ice in western fjords and a longer survival in uplands east of the Atlantic watershed.The significance of such a possibility for plant, animal and human coloni sation needs no stressing." documento, Del Coope (summarised in Pennington, 1974), working on beetle remains, noted that © early in Zone I (12,380-10,000 BC) there was a rapid rise in temperature, from less than 10° C as a July average to almost 17° C, though winters may have remained cold. -

Aberdeen Area, New Light on Old Coin Hoards . . 327-44 Aberdeen, Clarence Street, Coin Hoard ...328 Ross's Cour
Index Aberdeen coid arealighol w n n ne hoard,to 327-4. s 4 Cnoc Sligeach, Oronsay, Mesolithic site 3 ....2 , 17 . Aberdeen, Clarence Street, coin hoard .......... 328 Colonsay, new evidence for Mesolithic Ross's Court. Upperkirkgate, coin hoard in settlement ............................. 33-41 cauldron ........................ 328-9, 336-8 Druimvargie Cave, Mesolithic site .... 15, 17, 18-19 Shoe Lane, coin hoard ................ 331-20 ,34 SniachanDuc nMa , Early Historic sit 207. e , 209-11 St Nicholas Street, coin hoards ......... 327-80 33 , Dunadd, Early Historic site ............... 191-2, Mill of Maidencraig, Lang Stracht, coin 195,206-7,209-11,214 hoard ................. ............ 333,340-1 Dunollie, Early Historic site ............ 191, 195, ABERDEENSHIRE: 206,210-11,214,221 Aberdee ncoid area lighol n w n hoardne o ,t s 327-44 Duntealtaig, Colonsay, Mesolithic material Aberdeen see Aberdeen from ..................................... 37 Bridg Donf eo , coin hoard ............... 3305 33 , Gleann Mor, Islay, Mesolithic site ............. 33 Chapelden, Tor f Troupeo , excavatiof no Glengarrisdale, Jura, Mesolithic site ........... 35 beaker cist ................ 73-81, fiche l:Bl-8 Glenbatrick, Jura, Mesolithic site .... 12, 14, 16, 21 Valleye De , Mesolithi 23-, c 21 site4 , 19 s , ....14 , .4 Kilmelfort Cave, Mesolithic material from ..... 7-8 Footdee, coin hoards .............. 330-1, 339-40 Lealt Bay, Jura, Mesolithic site ......... 11-13, 16 Newhills, Bankhead Farm, coin hoards ..... 333-4, Loch Staosnaig, Colonsay, Mesolithic site .....5 3 . 340-1 Lower Kilchattan. Colonsay, Mesolithic Peterculter, Baads, coin hoard ............. 333-4 material .................................7 3 . Aberdour Castle, Fife, semicircular enam- Lussa Bay, Jura, Mesolithic site ......... 11-141 2 , elled mount ........................ 230,232-5 Lussa Riverjura, Mesolithic site ....... -
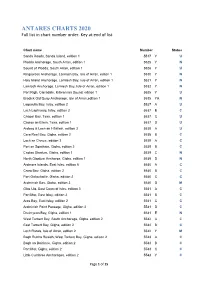
ANTARES CHARTS 2020 Full List in Chart Number Order
ANTARES CHARTS 2020 Full list in chart number order. Key at end of list Chart name Number Status Sanda Roads, Sanda Island, edition 1 5517 Y U Pladda Anchorage, South Arran, edition 1 5525 Y N Sound of Pladda, South Arran, edition 1 5526 Y U Kingscross Anchorage, Lamlash Bay, Isle of Arran, editon 1 5530 Y N Holy Island Anchorage, Lamlash Bay, Isle of Arran, edition 1 5531 Y N Lamlash Anchorage, Lamlash Bay, Isle of Arran, edition 1 5532 Y N Port Righ, Carradale, Kilbrannan Sound, edition 1 5535 Y U Brodick Old Quay Anchorage, Isle of Arran,edition 1 5535 YA N Lagavulin Bay, Islay, edition 2 5537 A U Loch Laphroaig, Islay, edition 2 5537 B C Chapel Bay, Texa, edition 1 5537 C U Caolas an Eilein, Texa, edition 1 5537 D U Ardbeg & Loch an t-Sailein, edition 3 5538 A U Cara Reef Bay, Gigha, edition 2 5538 B C Loch an Chnuic, edition 3 5539 A C Port an Sgiathain, Gigha, edition 2 5539 B C Caolas Gigalum, Gigha, edition 1 5539 C N North Gigalum Anchorge, Gigha, edition 1 5539 D N Ardmore Islands, East Islay, edition 5 5540 A C Craro Bay, Gigha, edition 2 5540 B C Port Gallochoille, Gigha, edition 2 5540 C C Ardminish Bay, Gigha, edition 3 5540 D M Glas Uig, East Coast of Islay, edition 3 5541 A C Port Mor, East Islay, edition 2 5541 B C Aros Bay, East Islay, edition 2 5541 C C Ardminish Point Passage, Gigha, edition 2 5541 D C Druimyeon Bay, Gigha, edition 1 5541 E N West Tarbert Bay, South Anchorage, Gigha, edition 2 5542 A C East Tarbert Bay, Gigha, edition 2 5542 B C Loch Ranza, Isle of Arran, edition 2 5542 Y M Bagh Rubha Ruaidh, West Tarbert -

Session 1 Time Travel in the Peak District from the Mesolithic to the Neolithic Aims of the Session Curriculum Links Resources
Stone Age to Iron Age KS2 Session 1 Time travel in the Peak District from the Mesolithic to the Neolithic Aims of the session The aim of this session is to explore the evidence of life in the local area from the Mesolithic to the Neolithic. Gather evidence in the gallery to create a video back at school about life in the Mesolithic times. Curriculum links This session will support pupils to develop a chronologically secure knowledge and understanding of British, local and world history, establishing clear narratives within and across the periods they study. They should note connections, contrasts and trends overtime and develop the appropriate use of historical terms. They should regularly address and sometimes devise historically valid questions about change, cause, similarity and difference, and significance. They should construct informed responses that involve thoughtful selection and organisation of relevant historical information. They should understand how our knowledge of the past is constructed from a range of sources. A visit to the Wonders of the Peak gallery will contribute to both an overview and a depth study to help pupils understand both the long arc of development and complexity of specific aspects of the content. Resources Handling collection, Stone Age Boy, DK Find out series, Stone Age Stone Age to Iron Age KS2 KS2 Session 1: 12,000 to 6,000 years ago The aim of this session is to explore the evidence of life in the local area from the Mesolithic to the Neolithic. Gather evidence in the gallery to create a video back at school about life in the Mesolithic times. -

Jottings on Some of the Objects of Interest in the Stonehenge Excursion
qass Jl/\ 142,- Book 'D 7 I- I . * JOTTINGS f)\' s<)\!!" m Tijy orjF^T*^ op I INTERKST ^ON 1 : 1 1 E N G E EXC U RS 1 N n\ EDWARD T. STEVENS. F.S.A Hon. Director of tlie, Salisbury and Souin Wilts Museum, Hon. C'jratoi- • m<* IV-.rstee th<? B-ricknioie Miiseuni, ; of trrespoiiding !SFember of the Academy of Nucural Sciences of Philadelphia, Forcicp. Member of ihc Anthropological Institute of New YorT<, A ..nor of Flint CKips," &c. SALISHUKN JOTTINGS ON SOME OF THE OBJECTS OF INTEREST IN THE TONEHENGE EXCURSION. BY EDWARD T. STEVENS, F.S.A., Hon. Director of the Salisbury and South Wilts Museum, Hon. Curator and Trustee of the Blackmore Museum, :orresponding y Member of the Academy of Natural Sciences of Philadelphia, Foreign Member of the Anthropological Institute of New York, Author of " Flint Chips," &c. I SALISBURY : BROWN & CO. LONDON : SIMPKIN, MARSHALL AND CO. 1882. V V ^^ .6 7 SALISBURY : BENNETT BROTHERS, PRINTERS, JOURNAL OFFICE. /WITHDRAWN N0Vl2:«18^ / CO^NTENTS. PAGE. Preface The Route I Traces of Early Occupation 2 Earth-works in the Route ... 2 Names of Rivers, &c., in the Route 3 Salisbury. —The Council Chamber ... 6 The Bull-ring 9 The Market-place II The " Blue Boar" 13 Ludlow and the Royalists H The City Gates 15 Events relating to Salisbury i6 Old Sarum. —General description 24 Remains of Masonry . 29 The Cathedral 30 Roman Roads to the Fortress 32 Palaeolithic Implement found there 33 Events relating to Old Sarum ... 34 Change in the name of the place 37 Removal of the Cathedral from Old Sarum 38 The Bishops of Old Sarum 39 Their remains and Tombs removed to Salisbury Cathedral 39 The Earls of Salisbury 43 The Burgh at Old Sarum 44 Represented in Parliament 44 Hour-glass stand in Stratford Church 46 Heale House. -
THE PLACE-NAMES of ARGYLL Other Works by H
/ THE LIBRARY OF THE UNIVERSITY OF CALIFORNIA LOS ANGELES THE PLACE-NAMES OF ARGYLL Other Works by H. Cameron Gillies^ M.D. Published by David Nutt, 57-59 Long Acre, London The Elements of Gaelic Grammar Second Edition considerably Enlarged Cloth, 3s. 6d. SOME PRESS NOTICES " We heartily commend this book."—Glasgow Herald. " Far and the best Gaelic Grammar."— News. " away Highland Of far more value than its price."—Oban Times. "Well hased in a study of the historical development of the language."—Scotsman. "Dr. Gillies' work is e.\cellent." — Frce»ia7is " Joiifnal. A work of outstanding value." — Highland Times. " Cannot fail to be of great utility." —Northern Chronicle. "Tha an Dotair coir air cur nan Gaidheal fo chomain nihoir."—Mactalla, Cape Breton. The Interpretation of Disease Part L The Meaning of Pain. Price is. nett. „ IL The Lessons of Acute Disease. Price is. neU. „ IIL Rest. Price is. nef/. " His treatise abounds in common sense."—British Medical Journal. "There is evidence that the author is a man who has not only read good books but has the power of thinking for himself, and of expressing the result of thought and reading in clear, strong prose. His subject is an interesting one, and full of difficulties both to the man of science and the moralist."—National Observer. "The busy practitioner will find a good deal of thought for his quiet moments in this work."— y^e Hospital Gazette. "Treated in an extremely able manner."-— The Bookman. "The attempt of a clear and original mind to explain and profit by the lessons of disease."— The Hospital. -

The Procurement of Rhum Bloodstone and the Rhum Bloodstone Exchange Network – a Social Territory in the Scottish Inner Hebrides?
The procurement of Rhum bloodstone and the Rhum bloodstone exchange network – a social territory in the Scottish Inner Hebrides? Torben Bjarke Ballin Abstract ‒ The purpose of the present paper is to discuss Rhum bloodstone and the distribution of archaeological bloodstone throughout the Scottish Inner Hebrides. The main aim is to discuss the observed distribution patterns, bloodstone procurement and exchange, and the social territory defined by bloodstone distribution. Key words ‒ archaeology; Inner Hebrides; Scotland; Rhum bloodstone; exchange networks; social territories; lithic raw material procure- ment; lithic raw material terminology Titel – Das Rohmaterial ‚Rhum Bloodstone‘: seine Beschaffung und sein Tausch-Netzwerk – Hinweis auf ein soziales Territorium auf den Inneren Hebriden (Schottland)? Zusammenfassung – Der Zweck des vorliegenden Aufsatz ist die Diskussion von “Rhum bloodstone” und die Verbreitung archäologi- scher Artefakte aus ‘bloodstone’ auf den Schottischen Hebriden. Dabei richtet sich der Fokus besonders auf erkannte Verteilungsmuster, die Beschaffung und den Austausch von ‘bloodstone’ und das durch die Verbreitung von ‘bloodstone’ festgelegte Sozialterritorium. Schlüsselwörter – Archäologie; Innere Hebriden; Schottland; Rhum bloodstone; Tauschnetzwerke; Sozialterritorien; Beschaffung von Steinrohmaterial; Terminologie von Steinrohmaterial Introduction What is Bloodstone – the basic lithic raw material terminology Since the publication of Wickham-Jones’ volume on the Kinloch site on the Isle of Rhum in the Scot- -

Platforms and a Lithic Scatter, Loch Doilean, Sunart, Lochaber by Clare Ellis (Argyll Archaeology) with Torben Bjarke Ballin and Susan Ramsay
ARO20: Activities in the woods: platforms and a lithic scatter, Loch Doilean, Sunart, Lochaber by Clare Ellis (Argyll Archaeology) with Torben Bjarke Ballin and Susan Ramsay Archaeology Reports Online, 52 Elderpark Workspace, 100 Elderpark Street, Glasgow, G51 3TR 0141 445 8800 | [email protected] | www.archaeologyreportsonline.com ARO20: Activities in the woods: platforms and a lithic scatter, Loch Doilean, Sunart, Lochaber Published by GUARD Archaeology Ltd, www.archaeologyreportsonline.com Editor Beverley Ballin Smith Design and desktop publishing Gillian McSwan Produced by GUARD Archaeology Ltd 2016. ISBN: 978-0-9928553-9-0 ISSN: 2052-4064 Requests for permission to reproduce material from an ARO report should be sent to the Editor of ARO, as well as to the author, illustrator, photographer or other copyright holder. Copyright in any of the ARO Reports series rests with GUARD Archaeology Ltd and the individual authors. The maps are reproduced by permission of Ordnance Survey on behalf of the Controller of Her Majesty’s Stationery Office. All rights reserved. GUARD Archaeology Licence number 100050699. The consent does not extend to copying for general distribution, advertising or promotional purposes, the creation of new collective works or resale. Contents Summary 6 Introduction and location 6 Archaeological background 6 Method of excavation 6 Raised terrace 6 Platforms 8 Results 8 Radiocarbon dates 8 Mesolithic 8 Late Iron Age? 10 Medieval platforms 10 Charcoal-burning platforms 17 Specialist reports 25 The lithic artefacts -

Pumice in the North Atlantic
Ocean-transported pumice in the North Atlantic Anthony Newton PhD University of Edinburgh 1999 Declaration I, Anthony Newton hereby declare that the work contained herein is my own and has not previously been presented for examination. Any contributions by others is acknowledged in the text. September 1999 ii For Keith Newton 1930-1984 iii “Some days we would pass through pumice lying in ridges, each piece uniformly the size and appearance of a bath sponge, then again we should pass through perfect fields of small yellow pumice spread evenly over the surface just for all the world like a green field of grass covered all over with buttercups, and the undulation of the swell of the trade wind produced an indescribably pretty appearance.” (Reeves, 1884). iv Abstract The overall aims of this study are to identify the sources of the widespread Holocene pumice deposits found along the coasts of the North Atlantic region and establish the ages of the source eruptions. In order to tackle this, it is necessary to determine whether it is possible to “fingerprint” the pumice of individual eruptions and link ocean-transported material with the established tephrochronological framework based on the stratigraphy of airfall deposits. Over 1500 electron probe microanalyses and over 200 Secondary Ion Mass Spectrometry analyses have been undertaken on pumice and tephra samples. These are the first high quality grain specific analyses carried out on ocean-transported pumice in the North Atlantic. Current knowledge of the extent of pumice distribution in the North Atlantic region is assessed for both shoreline (natural) and archaeological contexts. -

Swords, Fragments Of, At, . . . 364 Aberdeen Ca
INDEX Abbotsford, Bronze Mask from, fount a d Albany, Duke of, Plaster Cast of Panel Torrs, Kelton, (purchase) ..1 2 . with Arms of, from Old Trinity Hos- —— Swords, Fragments of, at, . 364 pital, (donation) ....9 1 . Aberdeen Cathedral, Vestment Tread an s - Alexander, James, ....6 5 , 54 . sure stored, sof Huntln i 7 y 15 Castle . , Allaman Bourgesf do , .... 7 f.n.7 —— Elphinstone, William, Bishop of, . 112 Allan, Jame elected, sH. , ...9 31 . —— King's College, ...... 161 Almon Tayd an d, Roman For t junctioa t n Aberdeenshire e Aberdourse : ; Aboyne; of, . .296,300 Auquharney: Cabrach (The); Clova; Amadeus VIII., Duke of Savoy : see Savoy, Coull; Da van; Gight, Braes of; Duk. eof Huntly Castle; Kildrummy; Lum- Amber Beads, Fragment , froof s m Glen phanan ; Migvie ; Rosehearty; Stand- Trool Hoard, (donation) ... 20 ingstones Farm Deerw Ne ,; Strath- Ancrum, Roxburghshire, Coped Stone bogie. at, .......8 18 . Aberdour, Aberdeenshire, Littoral Basins Anderson, Eric S., elected, .... 60 at, .......6 5 , 55 . 3 . 12 . — —. Grissel . , Aboyne, Aberdeenshire, 4 Famil5 , f 53 Bisse o y. t. — . — Helen . , at, ... f.n. l, 150 . RowandR — r —Si , Deat, hof —— Lordship of, ...... 154 —— —— Obituary notice of, . Account t Salvator'S f o s s Colleget S , —— Rev. R. S. G., on the Discovery of a Andrews, comprisin e Ordinarth g y Rock-Sculpture at Gallows Outon, Revenu Expenditured ean Casuae ,th l Whithorn, Wigtownshire, . 44 d Contingenan t Profits, etc., from Angus, William, elected, .... 2 1679 to 1689, and Details of the Animal Remains in Vessel of Pottery from Revenu 169n ei 1 ....5 32 . Traprain Law, .... -

9. Bibliography for Research Agenda (Pdf)
The Archaeology of North West England Bibliography Abramson P, 2000, ‘A re-examination of a Viking Age burial at trial Archaeology Review 27(1), 113-120. Beacon Hill, Aspatria’, TTCWAAS 100, 79-88. Barnwell P S, Giles C, 1997, English farmsteads: 1750-1914. Swin- Adams M H, 1995, An archaeological evaluation at St Chad’s Church, don: RCHME. Kirkby, Knowsley, Merseyside. Liverpool Museum unpublished Barrett J, 1999a, ‘The mythical landscapes of the British Iron report. Age’, in Ashmore W & Knapp A B, (eds), Archaeologies of Abrams L & Parsons D N, 2004, ‘Place-names and the history of landscape, 253-265. Oxford: Blackwell. Scandinavian settlement in England’, in Hines J, Lane A & Barrett J, 1999b, ‘Chronologies of landscape’, in Layton R & Redknap M, (eds), Land, sea and home: proceedings of a conference Ucko P, (eds), The archaeology and anthropology of landscape, 21- on Viking age settlement, Cardiff 2001, 379-431. Leeds: Society 30. London: Routledge. Medieval Archaeology Monograph 20. Barrett J, Locker A & Roberts C, 2004, ‘The origins of intensive ADCA, 2004, Archaeological requirements for works on churches and marine fishing in medieval Europe: the English evidence’, churchyards. Association of Diocesan and Cathedral Archae- published online in Proceedings of the Royal Society B, 24 Novem- ologists Guidance Note 1. ber 2004. Albarella U, 2003, ‘Tawyers, tanners, horn trade and the mystery Barrow G W S, 1969, ‘Northern English society in the Early of the missing goat’, in Murphy P & Wiltshire P E J, (eds), Middle Ages’, N.H. 4 1-28. The environmental archaeology of industry, 71-83. Oxford: Oxbow. Barter M, 2004, ‘The Diocese of Manchester: strategy, capacity Alcock L, 1972, ‘By South Cadbury is that Camelot . -

SCOTTISH ARCHAEOLOGICAL INTERNET REPORTS E-ISSN: 2056-7421
SCOTTISH ARCHAEOLOGICAL INTERNET REPORTS e-ISSN: 2056-7421 Mesolithic and later activity at North Barr River, Morvern How to cite: MacGregor, G 2019 ‘Mesolithic and later activity at North Barr River, Morvern’. Scottish Archaeological Internet Reports 84 https://doi.org/10.9750/issn.2056-7421.2019.84 Visit http://journals.socantscot.org/index.php/sair to see the journal homepage Please note: This document is the publisher’s PDF of an article published in the Scottish Archaeological Internet Reports journal. This version has been peer- reviewed and contains all final editorial corrections and journal pagination. Copyright © 2019 rests with the Society and the individual authors. Except where otherwise noted, this work is licensed under Creative Commons Attribution-NonCommercial-No Derivatives licence. http://creativecommons.org/licenses/by-nc-nd/4.0/ The permission to reproduce the Society's copyright-protected material does not extend to any material which is identified as being the copyright of a third party. Authorisation to reproduce such material must be obtained from the copyright holders concerned. Mesolithic and later activity at North Barr River, Morvern Gavin MacGregor with contributions by Alistair Becket, Ann Clarke, Nyree Finlay, David Sneddon and Jennifer Miller Address Northlight Heritage, Studio 114, South Block, 64 Osborne Street, Glasgow, G1 5QH Author contact [email protected] Funding Forestry and Land Scotland e-ISSN: 2056-7421 https://doi.org/10.9750/issn.2056-7421.2019.84 Published by the Society of Antiquaries of Scotland. Society of Antiquaries of Scotland National Museums Scotland Chambers Street Edinburgh EH1 1JF United Kingdom Managing editor: Catherine Aitken Copy-editor: Helen Bleck Production: Raspberry Creative Type, Edinburgh Copyright © 2019 Gavin MacGregor and individual contributors.