A NOVEL GAIN of FUNCTION of the <I>IRX1</I> and <I>IRX2</I> GENES DISRUPTS AXIS ELONGATION in the ARAUCA
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ameraucana Handbook 2014
Ameraucana Breeders Club Handbook - 2014 Ameraucana Breeders Club Handbook Fourth Edition - January 2014 rauc e an m a A Breeders Club Ameraucana.org The ABC Logo, drawing on cover and sketches on page 8 are by Rusty Hart, of Michigan Ameraucana Breeders Club All Rights Reserved. A Non-Profit Organization. Ameraucana Bantam Handbook - 1982 Ameraucana Breeders Handbook - 1998 Ameraucana Breeders Handbook - 2005 1 Contents Foreword Foreword.................................................................................. 3 Yet another edition of the Ameraucana Breeders Club Handbook has Constitution & Bylaws................................................................ 4 been depleted. Progress – and time – marches on. Looking back over 40 years of work with the blue egg bantam that came to be known as Ameraucana, we Map of Districts......................................................................... 7 realize that not all what was planned and hoped for was accomplished. Yet so Officers..................................................................................... 8 much has come to fruition that there can be no regrets. We can look with satisfaction upon the general acceptance and worldwide recognition of that What Is A Poultry Breeder?........................................................10 special convergence of characteristics that comprise what we today know as the The Art of Cage Training...........................................................12 Ameraucana breed of poultry - in both large fowl and bantam. History -

Snake Skeletonizing Manual
Snake Skeletonizing Manual By Ellen Kuo Illustrations by Omar Malik, Juliana Olsson, and Sara Brenner © 2020 Museum of Vertebrate Zoology Table of Contents Snake anatomy reference images …………………………………………………. Page 2-3 Station setup …………………………………………………. Page 4 Initial data collection and setup …………………………………………………. Page 5-8 Taking photos …………………………………………………. Page 9 Initially determining the sex ………………………………………………… Page 9-10 Skinning …………………………………………………. Page 11-12 Opening and sexing …………………………………………………. Page 13-24 Examples of male gonads …………………………………………………. Page 14-16 Examples of female gonads …………………………………………………... Page 17-23 Taking tissues …………………………………………………. Page 25 Stomach contents, parasites …………………………………………………. Page 25 Finishing and cleaning up …………………………………………………. Page 26 1 Snake Anatomy References 2 Illustration by Sara Brenner Snake skeleton – note that the ribs go down the whole length of the body (they end at the vent, and then the tail does not have ribs). Illustration by Sara Brenner Most snake skulls consist of many small, delicate bones that are unfused. The lower jaw is not fused at the center, allowing the snake to use its lower jaws like arms to slowly feed in prey. Snakes have very sharp, delicate teeth, and lots, and lots, and lots of them — typically on several different jaw bones! Avoid disturbing the teeth. 3 Station Setup Materials ● Snake ● Original data ● Skeleton tag ● Gloves ● Worksheet ● Micron pen ● Forceps ● Scissors (large and small) ● Tray (optional) ● Camera* ● Ruler and/or measuring tape ● Tissue vial ● Vial pen* ● MVZ barcode (for tissue vial) ● Paper towel labeled with H, L, M, K ● Prep Lab Catalog* ● Extra paper towels (optional) ● Scale* ● Herp field guide (for local animals)* ● Probe ● Biohazard bin* *shared materials with the rest of the class 4 Before you start cutting ● Set up your station with all of the listed materials (or access to them) ● Identify the genus and species of your specimen, double checking with the class coordinator to make sure it is correct. -

Kansas Herpetological Society Newsletter No. 54 December, 1983
KANSAS HERPETOLOGICAL SOCIETY NEWSLETTER NO. 54 DECEMBER, 1983 1984 KHS DUES DUE, DO ... In this issue of the KHS Newsletter, you should find the nifty return-by-mail envelope for payment of your 1984 Kansas Herpetological Society dues. Since your dues are what finances this newsletter, prompt payment is appreciated. If you have already paid your 1984 dues, pass the envelope on to a friend who would like to JO~n the Kansas Herpetological Society. Of all the Regional Herpetological Societies in the U.S . , the KHS has some of the LO\fEST membership rates. If you are missing your dues envelope, or have lost it, the rates are still as follows: Regular member (U.S . ) $4.00 Non-U .S. member $8.00 Contributing member $15.00 Make your checks or money orders payable to KHS. Be sure that your CORRECT mailing address is printed neatly on the outside of the envelope. Send your money to: Kansas Herpetological Society Museum of Natural History University of Kansas Lawrence, Kansas 66045 KHS NEWSLETTER NO. 54 1 ANNOUNCENENTS Who Are Those Herpetologists, Anyway? If you are in the mood to expand your Christmas card list, here is a chance to get the names and addresses of over 2,000 professional and amateur herpetologists, plus lots of other neat stuff. The Silver Anniversary Membership Directory of the Society for the Study of Amphibians and Reptiles has just been published and also contains a list of herpetological societies and organizations of the world (organized by country, each listing includes an address to write to and usually a list of publications) , a brief history of the SSAR, and other useful information about the SSAR and its organization. -

Classification of the Major Taxa of Amphibia and Reptilia
Station 1. Amphibian and Reptile Diversity Classification of the Major Taxa of Amphibia and Reptilia ! Phylum Chordata examples ! Subphylum Vertebrata ! Class Amphibia ! Subclass Labyrinthodontia extinct earliest land vertebrates ! Subclass Lepospondyli extinct forms of the late Paleozoic ! Subclass Lissamphibia modern amphibians ! Order Urodela newts and salamanders ! Order Anura frogs and toads ! Order Gymnophiona caecilians ! Class Reptilia ! Subclass Anapsida ! Order Captorhinomorpha extinct stem reptiles ! Order Testudina (Chelonia) turtles ! Subclass Synapsida ! Order Pelycosauria primitive mammal-like reptiles ! Order Therapsida advanced mammal-like reptiles ! Subclass Lepidosaura ! Order Eosuchia early lepidosaurs ! Order Squamata lizards, snakes, amphisbaenians, and the tuatara ! Subclass Archosauria ! Order Thecodontia extinct ancestors of dinosaurs, birds, etc ! Order Pterosauria extinct flying reptiles ! Order Saurischia dinosaurs with pubis extending anteriorly ! Order Ornithischia dinosaurs with pubis rotated posteriorly ! Order Crocodilia crocodiles and alligators ! Subclass Euryapsida extinct marine reptiles Station 1. Amphibian Skin AMPHIBIAN SKIN Most amphibians (amphi = double, bios = life) have a complex life history that often includes aquatic and terrestrial forms. All amphibians have bare skin - lacking scales, feathers, or hair -that is used for exchange of water, ions and gases. Both water and gases pass readily through amphibian skin. Cutaneous respiration depends on moisture, so most frogs and salamanders are -

Biological System Models Reproducing Snakes’ Musculoskeletal System
The 2010 IEEE/RSJ International Conference on Intelligent Robots and Systems October 18-22, 2010, Taipei, Taiwan Biological System Models Reproducing Snakes’ Musculoskeletal System Kousuke Inoue, Kaita Nakamura, Masatoshi Suzuki, Yoshikazu Mori, Yasuhiro Fukuoka and Naoji Shiroma Abstract— Snakes are very unique animals that have dis- including the interaction in order to elucidate the mechanism tinguished motor function adaptable to the most diverse en- of emerging animals’ adaptive motor functions. vironments in terrestrial animals regardless of their simple cord-shaped body. Revealing the mechanism underlying this B. Previous works on snakes’ locomotion mechanisms distinct locomotion pattern, which is fundamentally different from walking, is signifficant not only in biolgical field but The researches on snakes in biology have been conducted also for applications in engineering firld. However, it has mainly on taxonomy, anatomy and snake poison and there been difficult to clarify this adaptive function, emerging from are few researches on snake locomotion until now. In lo- dynamic interaction between body, brain and environment, comotion studies [1]-[15], analytical discussions have been by previous scientific methodologies based on reductionism, carried out based on kinematics recording with respect to where understanding of the total system is approached by analyzing specific individual elements. In this research, we aim specific locomotion modes or EMG recording with a few at revealing the mechanisms underlying this adaptability by muscles that are said to be dominant for locomotion. For the use of the constructive methodology, in which biological example, Jayne [10] records EMG with the three dominant system models reflecting biological knowledge is used as a tool muscles with lateral undulation locomotion in terrestrial and for analysis of the total system. -

Serpent of the Southeast the Orianne Society’S Efforts to Conserve Eastern Diamondback Rattlesnake Populations
The Member Magazine of The Orianne Society Issue 1 • Spring 2013 Indigomagazine our giant Serpent of the southeast The Orianne Society’s Efforts to Conserve Eastern Diamondback Rattlesnake Populations Also in this issue: Travelers Backwoods of the The Complexity Blackwater Snake of Conserving Canoeing Georgia’s The Eastern Suwannee River World Indigo Snake photo: Pete Oxford Pete photo: Indigomagazine 14 Our Giant Serpent of the Southeast The Orianne Society’s Efforts to Conserve Eastern Diamondback Rattlesnake Populations photo: Pete Oxford Pete photo: Herper 6 Events 44 Field 46 Spotlight Calendar Photos Two young herp Our events calendar If you like us on Facebook enthusiasts share their has a wide selection of you know that we get great passion for snakes and snake and reptile focused photos submitted to us snake conservation. The events from around the daily. We pulled together future is bright for the country. Find an event some of our favorites in our field of Herpetology. near you. Field Photos. Too bad we didn’t have room for more. 2 ORIANNESOCIETY.ORG SPRING ISSUE 2013 Indigomagazine Reminiscences of 8a Snake Hunter Dirk Stevenson reflects on a lifetime of snake hunting, from boyhood excursions to his work as a biologist. staff Christopher L. Jenkins CEO What’s the Frederick B. Antonio Director of OCIC 31Frequency, Betty? Wayne O. Taylor Director of Land Management Using radio telemetry to track Indigos Stephen F. Spear Director of Amphibian with The Orianne Society in Central Conservation Florida. Heidi L. Hall Director of Communications Dirk Stevenson Director of Inventory and Monitoring Javan M. Bauder Assistant Conservation Scientist Backwoods, Patrick Barnhart Indigo Snake Technician 40Blackwater Sue Bottoms Administrative Assistant Canoeing the blackwater of the Polly Conrad Communication Specialist - Suwannee River. -

An Overview of Pet Reptile Species and Proper Hand
Exotics — Reptiles and Amphibians ______________________________________________________________________________________________ AN OVERVIEW OF PET REPTILE SPECIES Many reptiles are still caught from the wild, and AND PROPER HANDLING shipped to distributors who then supply pet stores. For one animal that makes it through that journey, many if Jean A. Paré, DMV, DVSc, Diplomate ACZM not most, will die. Sometimes whole shipments are lost. College of Veterinary Medicine Wild-caught animals are often fractious and do not adapt Texas A&M University, College Station, TX well to captivity. They come with a parasite burden that may well become overbearing with the stress and the Reptiles are a successful group of ectothermic, scaled confinement of captivity. Not only does buying a captive- vertebrates that are present on all continents except bred reptile help discourage the wild-caught trade, but Antarctica. Taxonomic debates are ongoing and new captive-bred animals are better-adapted, accept reptile species are discovered every year, but it is a handling much better, are less finicky eaters, have fewer reasonable estimate that there are over 7,500 extant parasites, and live longer and healthier lives as a rule species of reptiles, among which roughly 4,500 are than wild-caught specimens. It is incumbent upon us to lizards, 3,000 are snakes, 300 are turtles, 23 are advise potential reptile owners to seek captive-bred crocodilians, and 2 species of tuataras. The world being animals. Some animals are sold under misleading what it is, with the dwindling of habitats and the appellations, such as “farm-raised” or “captive-raised” increasing encroachment of humans on the remaining reptiles, which may only mean that they were maintained wilderness, there are numerous species of reptiles that captive (for weeks to months) after being caught in the are threatened or endangered, often critically. -
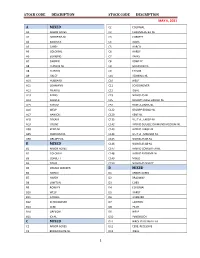
Stock Codes Numerical
STOCK CODE DESCRIPTION STOCK CODE DESCRIPTION MAY 6, 2021 A MIXED C2 COLONIAL A1 ARBOR ACRES C3 CHAUMIERE BB-NL A2 ANDREWS-NL C3 CORBETT A2 BABCOCK C4 DAVIS A3 CAREY C5 HARCO A5 COLONIAL C6 HARDY A6 EURIBRID C7 PARKS A7 GARBER C8 ROWLEY A8 H AND N-NL C9 GUILFORD-NL A8 H AND N C9 TATUM A9 HALEY C10 HENNING-NL A10 HUBBARD C10 WELP A11 LOHMANN C11 SCHOONOVER A12 MERRILL C12 IDEAL A13 PARKS C19 NICHOLAS-NL A14 SHAVER C35 ORLOPP LARGE BROAD-NL A15 TATUM C57 ROSE-A-LINDA-NL A16 WELP C122 ORLOPP BROAD-NL A17 HANSON C129 KENT-NL A18 DEKALB C135 B.U.T.A., LARGE-NL A19 HYLINE C142 HYBRID DOUBLE DIAMOND MEDIUM-NL A38 KENT-NL C143 HYBRID LARGE-NL A45 MARCUM-NL C144 B.U.T.A., MEDIUM-NL A58 ORLOPP-NL C145 NICHOLAS 85-NL B MIXED C146 NICHOLAS 88-NL B1 ARBOR ACRES C147 HYBRID CONVERTER-NL B2 COLONIAL C148 HYBRID EXTREME-NL B3 CORBETT C149 MIXED B4 DAVIS C150 NICHOLAS SELECT B5 DEKALB WARREN D MIXED B6 HARCO D1 ARBOR ACRES B7 HARDY D2 BRADWAY B8 LAWTON D3 COBB B9 ROWLEY D4 COLONIAL B10 WELP D5 HARDY B11 CARGILL D6 HUBBARD B12 SCHOONOVER D7 LAWTON B13 CEBE D8 PILCH B14 OREGON D9 WELP B15 IDEAL D10 PENOBSCOT C MIXED D11 WROLSTAD SMALL-NL C1 ARBOR ACRES D11 CEBE, RECESSIVE C2 BROADWHITE-NL D12 IDEAL 1 STOCK CODE DESCRIPTION STOCK CODE DESCRIPTION MAY 6, 2021 N14 OLD ENGLISH, WHITE E MIXED N15 OLD ENGLISH, BLACK E1 COLONIAL N16 OLD ENGLISH, SPANGLED E2 HUBBARD N17 PIT E3 BOURBON, RED-NL N18 OLD ENGLISH E3 ROWLEY N19 MODERN E4 WELP N20 PIT, WHITE HACKLE E5 SCHOONOVER N21 SAM BIGHAM E6 CEBE N22 MCCLANHANS H MIXED N23 CLIPPERS H1 ARBOR ACRES N24 MINER BLUES H2 -

Design and Analysis of a Bio-Inspired Wire-Driven Multi-Section Flexible Robot
ARTICLE International Journal of Advanced Robotic Systems Design and Analysis of a Bio-Inspired Wire-Driven Multi-Section Flexible Robot Regular Paper Zheng Li1,* and Ruxu Du1 1 Institute of Precision Engineering, Dept. Mechanical & Automation Engineering, The Chinese University of Hong Kong, Hong Kong * Corresponding author E-mail: [email protected] Received 24 Jul 2012; Accepted 31 Jan 2013 DOI: 10.5772/56025 © 2013 Li and Du; licensee InTech. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract This paper presents a bio‐inspired wire‐driven 1. Introduction multi‐section flexible robot. It is inspired by the snake skeleton and octopus arm muscle arrangements. The In the field of robotics, researchers have been building robot consists of three sections and each section is made robots that mimic the appearance or function of their up of several identical vertebras, which are articulated by natural counterparts, such as serpentine robots and both spherical joints and a flexible backbone. Each section continuum robots. A Serpentine robot has a similar is driven by two groups of wires, controlling the bending structure to the snake skeleton. It is composed of multiple motion in X and Y directions. This design integrates the links, with each link driven by a servomotor [1] or other serpentine robots’ structure and the continuum robots’ actuators, e.g., pneumatic artificial muscles [2]. The idea actuation. As a result, it is more compact than traditional of a continuum robot comes from the octopus’ arm, serpentine robots and has a higher positioning accuracy elephant’s trunk, mammal’s tongue, etc. -

A Fossil Snake (Elaphe Vulpina) from a Pliocene Ash Bed in Nebraska J
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Transactions of the Nebraska Academy of Sciences Nebraska Academy of Sciences and Affiliated Societies 1982 A Fossil Snake (Elaphe vulpina) From A Pliocene Ash Bed In Nebraska J. Alan Holman Michigan State University Follow this and additional works at: http://digitalcommons.unl.edu/tnas Holman, J. Alan, "A Fossil Snake (Elaphe vulpina) From A Pliocene Ash Bed In Nebraska" (1982). Transactions of the Nebraska Academy of Sciences and Affiliated Societies. 492. http://digitalcommons.unl.edu/tnas/492 This Article is brought to you for free and open access by the Nebraska Academy of Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Transactions of the Nebraska Academy of Sciences and Affiliated Societies by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. 1982. Transactions of the Nebraska Academy ofSciences, X:37-42. A FOSSIL SNAKE (ELAPHE VULPINA) FROM A PLIOCENE ASH BED IN NEBRASKA J. Alan Holman Museum Michigan State University East Lansing, Michigan 48824 The articulated skeleton of a fossil snake from the late Middle occipital, quadrates, parasphenoid, basisphenoid, splenials, Pliocene of northeastern Nebraska is unique in that it is one of the most dentaries, angulars, articulars, supra-angulars, and coronoids. complete fossil snakes known; it was preserved by an ash-fall. It is iden The other skull elements crushed beyond recognition. Post tified as the modern species Ekzphe vulpina, and it appears to have been trampled by a large ungulate. cranial elements: 47 cervical vertebrae, 146 trunk vertebrae, 46 caudal vertebrae, and 155 ribs. -

On Archaeophis Proavus Mass
1 ON ARCHAEOPHIS PROAVUS MASS., A SNAKE FROM THE EOCENE OF MONTE BOLCA by Dr. W. Janensch. Über Archaeophis proavus Mass., eine schlange aus dem Eocän des Monte Bolca. Beiträge zur Paläontologie und Geologie Oestreich-Ungarns und des Orients 19: 1-33, Pl.I-II (1906) (Trans. Ó2000 John D. Scanlon, Department of Zoology, University of Queensland, Brisbane QLD 4072, Australia) Table of contents Page (original , translation) Introduction 1 2 A. The Skull. Description of the parts present. 2 3 Reconstruction of the jaw apparatus. 5 6 The dentition. 6 7 B. Vertebrae. Preservation. 7 9 Number of vertebrae and length of the vertebral column. 8 10 The size proportions of the vertebrae. 8 11 Presacral vertebrae. 9 12 Postsacral vertebrae. 11 14 The ribs. 11 14 Extremities. 13 17 The squamation. 13 17 The external body form and way of life of Archaeophis. 15 19 Comparison with Archaeophis bolcensis Mass. 17 23 Degree of specialisation of Archaeophis and comparison with living water snakes. 19 25 Systematic position of the genus Archaeophis. 24 31 On the descent of snakes. 26 34 Summary of most important results. 31 40 2 1 Introduction The geologisch-paläontologisch Museum der Berliner Universität a short time ago came into possession of a fossil snake that came from the Eocene limestones of Monte Bolca, well known to be rich in fossils, especially splendid fish, and had been in the collection of Herzog of Canossa. In a work which was not widely distributed and which, as a consequence, until now had not been cited in our usual textbooks of palaeontology, Massalongo (Specimen photographicum animalium quorundam plantarumque fossilium agri Veronensis, 1849) already more than half a century ago described this snake as Archaeophis proavus, and together with it the fragments of a second, much larger form which received the name Archaeophis bolcensis. -

Bioarchaeology in Southeast Asia and the Pacific: Newsletter
Bioarchaeology in Southeast Asia and the Pacific: Newsletter Issue No 6 April 2010 Edited by Kate Domett [email protected] Welcome to the sixth annual newsletter designed to update you on the latest news in the field of bioarchaeology in Southeast Asia and the Pacific. Please circulate to your colleagues and students and email me if you wish to be added to the email recipient list. News WESTERN PACIFIC From: Professor Michael Pietrusewsky University of Hawai’i Email: [email protected] Subject: Fieldwork in the Mariana Islands Currently Michael Pietrusewsky is examining 12 skeletons from the Tinian Route 202, Tinian, CNMI (Commonwealth of the Northern Mariana Islands), for Swift and Harper Archaeological Resource Consulting. July 2009: Michael Pietrusewsky, Michele Toomay Douglas, Moana Lee, Rona Ikehara, Joey Condit and Karen Kadohiro examined human skeletal remains from the Ylig Bay archaeological site, Yona Bay, Guam or International Archaeological Research Institute, Inc. Report: The Osteology of the human skeletons from the Ylig Bay archaeological site (66-09-1872), Yona, Guam. Final Report Prepared for: Parsons Transportation Group (PTG) and the Guam Department of Public Works (DPW) under Federal Highway Administration (FHWA), Archaeological Salvage, Data Recovery, Burial Recovery, Monitoring, and Mitigation of the Ylig Bay Archaeological Site. International Archaeological Research Institute, Inc. Michael Pietrusewsky, Michele Toomay Douglas and Rona M. Ikehara-Quebral, 2009. ABSTRACT: Human skeletal material from at least fifty-three burials excavated during the archaeological salvage, data recovery, burial recovery, monitoring, and mitigation of the Ylig Bay archaeological site (66-09-1872) for Parsons Transportation Group (PTG) and the Guam Department of Bioarchaeology in Southeast Asia and the Pacific: Newsletter Issue 6, April 2010 Public Works (DPW) under Federal Highway Administration (FHWA) at Ylig Bay, Guam are described.