Artículos Científicos
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Safety of Immunization During Pregnancy a Review of the Evidence
Safety of Immunization during Pregnancy A review of the evidence Global Advisory Committee on Vaccine Safety © World Health Organization 2014 All rights reserved. Publications of the World Health Organization are available on the WHO website (www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications –whether for sale or for non-commercial distribution– should be addressed to WHO Press through the WHO website (www.who.int/about/licensing/copyright_form/en/index.html). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. -

Vaccines for Preteens
| DISEASES and the VACCINES THAT PREVENT THEM | INFORMATION FOR PARENTS Vaccines for Preteens: What Parents Should Know Last updated JANUARY 2017 Why does my child need vaccines now? to get vaccinated. The best time to get the flu vaccine is as soon as it’s available in your community, ideally by October. Vaccines aren’t just for babies. Some of the vaccines that While it’s best to be vaccinated before flu begins causing babies get can wear off as kids get older. And as kids grow up illness in your community, flu vaccination can be beneficial as they may come in contact with different diseases than when long as flu viruses are circulating, even in January or later. they were babies. There are vaccines that can help protect your preteen or teen from these other illnesses. When should my child be vaccinated? What vaccines does my child need? A good time to get these vaccines is during a yearly health Tdap Vaccine checkup. Your preteen or teen can also get these vaccines at This vaccine helps protect against three serious diseases: a physical exam required for sports, school, or camp. It’s a tetanus, diphtheria, and pertussis (whooping cough). good idea to ask the doctor or nurse every year if there are any Preteens should get Tdap at age 11 or 12. If your teen didn’t vaccines that your child may need. get a Tdap shot as a preteen, ask their doctor or nurse about getting the shot now. What else should I know about these vaccines? These vaccines have all been studied very carefully and are Meningococcal Vaccine safe. -

Meningococcal Vaccine Q & a for Healthcare Providers
Meningococcal Vaccine Q & A for Healthcare Providers School meningococcal vaccine requirements Q1: When did the school meningococcal vaccine requirement take effect? A1: The meningococcal vaccine school requirement took effect on September 1, 2016. Q2: For what grades is meningococcal vaccine required? A2: Meningococcal vaccine is currently required for students entering or attending grades 7 through 12 in public, private and parochial New York State (NYS) schools. Q3: How many doses of meningococcal vaccine are required for grades 7 through 11? A3: One dose of meningococcal conjugate vaccine (MenACWY; sometimes abbreviated as MCV4; brand names Menactra or Menveo) is required for entry into grades 7 through 11. Q4: How many doses of meningococcal vaccine are required for grade 12? A4: A total of two doses of MenACWY vaccine, administered a minimum of 8 weeks apart, are required for entry into grade 12. The second dose must be administered no sooner than 16 years of age. However, if the first dose of MenACWY vaccine was received at 16 years of age or older, then a second dose will not be required. The NYS school immunization requirements allow for a grace period of up to 4 days before the 16th birthday for receipt of the dose. A dose of vaccine received 5 or more days before the 16th birthday will not meet the 12th grade meningococcal vaccine requirement. Q5: Is serogroup B meningococcal vaccine (MenB vaccine) required for grade 12? A5: No, MenB vaccine is not required for school attendance in NYS. In addition, doses of MenB vaccine will not meet the NYS MenACWY vaccine requirement. -

ABSTRACT FROMEN, CATHERINE ANN. Monodisperse, Uniformly
ABSTRACT FROMEN, CATHERINE ANN. Monodisperse, Uniformly-Shaped Particles for Controlled Respiratory Vaccine Delivery. (Under the direction of Joseph M. DeSimone). The majority of the world’s most infectious diseases occur at the air-tissue interface called the mucosa, including HIV/AIDS, tuberculosis, measles, and bacterial or viral gut and respiratory infections. Despite this, vaccines have generally been developed for the systemic immune system and fail to provide protection at the mucosal site. Vaccine delivery directly to the lung mucosa could provide superior lung protection for many infectious diseases, such as TB or influenza, as well as systemic and therapeutic vaccines for diseases such as Dengue fever, asthma, or cancer. Specifically, precision engineered biomaterials are believed to offer tremendous opportunities for a new generation of vaccines. The goal of this approach is to leverage naturally occurring processes of the immune system to produce memory responses capable of rapidly destroy virulent pathogens without harmful exposure. Considerable knowledge of biomaterial properties and their interaction with the immune system of the lung is required for successful translation. The overall goal of this work was to fabricate and characterize nano- and micro- particles using the Particle Replication In Non-wetting Templates (PRINT) fabrication technique and optimize them as pulmonary vaccine carriers. The main objectives of this PhD research included (1) the development of a calibration-quality aerosol system using PRINT, the application of these calibration-quality aerosols to improve understanding of (2) shaped aerosols under flow and (3) their cellular fate in the lung, and (4) the application of this knowledge towards the development of a mucosal vaccine. -

PROOF of IMMUNIZATION COMPLIANCE NORTHWESTERN STATE UNIVERSITY of LOUISIANA (Louisiana R.S
PROOF OF IMMUNIZATION COMPLIANCE NORTHWESTERN STATE UNIVERSITY OF LOUISIANA (Louisiana R.S. 17:170.1 Schools of Higher Learning) SS Number: _____________________________________________ Date of Birth: Month _________________ Date ___________________ Year ________________ Name: __________________________________________________________________________________________________________________________________ Please Print (Last) (First) (Middle) Address: ________________________________________________________________________________________________________________________________ City: ______________________________________________________ State: ________________________________ ZIP Code: _____________________________ UNIVERSITY REQUIRED IMMUNIZATIONS: Physician or Other Health Care Provider Verification: (See other side) M-M-R (Measles, Mumps, Rubella-2 Doses Required) Tetanus Diphtheria (Td) Pertussis (Tdap) OR First dose: ___________________ Serologic Test: __________________ Td: ___________________ (Date) (Date within 10 years) (Date) OR Second dose: __________________ (Date) Result: _________________________ (Date) Tdap: ___________________ (Date within 10 years) OR □ Born before 1956 Meningitis Vaccine ACYW-135 (TWO doses of meningococcal conjugate vaccination separated by at least eight weeks.) First dose: ____________________________________ Vaccine Type: _______________________________________ (Date) Second dose: __________________________________ Vaccine Type: _______________________________________ (Date) UNIVERSITY REQUIRED IMMUNIZATIONS: -

Global Vaccine Action Plan Monitoring, Evaluation & Accountability Secretariat Annual Report 2016 © World Health Organization 2016
Global Vaccine Action Plan Monitoring, Evaluation & Accountability Secretariat Annual Report 2016 © World Health Organization 2016 All rights reserved. Publications of the World Health Organization are available on the WHO website (www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications –whether for sale or for non-commercial distribution– should be addressed to WHO Press through the WHO website (www.who.int/about/licensing/copyright_form/en/index.html). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate borderlines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. The responsibility for the interpretation and use of the material lies with the reader. -

COVID-19 Vaccines: Summary of Current State-Of-Play Prepared Under Urgency 21 May 2020 – Updated 16 July 2020
Office of the Prime Minister’s Chief Science Advisor Kaitohutohu Mātanga Pūtaiao Matua ki te Pirimia COVID-19 vaccines: Summary of current state-of-play Prepared under urgency 21 May 2020 – updated 16 July 2020 The COVID-19 pandemic has spurred a global effort to find a vaccine to protect people from SARS- CoV-2 infection. This summary highlights selected candidates, explains the different types of vaccines being investigated and outlines some of the potential issues and risks that may arise during the clinical testing process and beyond. Key points • There are at least 22 vaccine candidates registered in clinical (human) trials, out of a total of at least 194 in various stages of active development. • It is too early to choose a particular frontrunner as we lack safety and efficacy information for these candidates. • It is difficult to predict when a vaccine will be widely available. The fastest turnaround from exploratory research to vaccine approval was previously 4–5 years (ebolavirus vaccine), although it is likely that current efforts will break this record. • There are a number of challenges associated with accelerated vaccine development, including ensuring safety, proving efficacy in a rapidly changing pandemic landscape, and scaling up manufacture. • The vaccine that is licensed first will not necessarily confer full or long-lasting protection. 1 Contents Key points .................................................................................................................................. 1 1. Types of vaccines ............................................................................................................... -
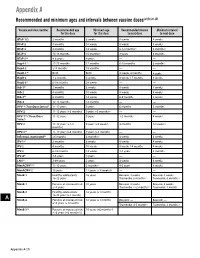
Recommended and Minimum Ages and Intervals Between Doses
Appendix A Recommended and minimum ages and intervals between vaccine doses(a),(b),(c),(d) Vaccine and dose number Recommended age Minimum age Recommended interval Minimum interval for this dose for this dose to next dose to next dose DTaP-1(e) 2 months 6 weeks 8 weeks 4 weeks DTaP-2 4 months 10 weeks 8 weeks 4 weeks DTaP-3 6 months 14 weeks 6-12 months(f) 6 months(f) DTaP-4 15-18 months 15 months(f) 3 years 6 months DTaP-5(g) 4-6 years 4 years — — HepA-1(e) 12-23 months 12 months 6-18 months 6 months HepA-2 ≥18 months 18 months — — HepB-1(h) Birth Birth 4 weeks-4 months 4 weeks HepB-2 1-2 months 4 weeks 8 weeks-17 months 8 weeks HepB-3(i) 6-18 months 24 weeks — — Hib-1(j) 2 months 6 weeks 8 weeks 4 weeks Hib-2 4 months 10 weeks 8 weeks 4 weeks Hib-3(k) 6 months 14 weeks 6-9 months 8 weeks Hib-4 12-15 months 12 months — — HPV-1 (Two-Dose Series)(l) 11-12 years 9 years 6 months 5 months HPV-2 11-12 years (+6 months) 9 years +5 months(m) — — HPV-1(n) (Three-Dose 11-12 years 9 years 1-2 months 4 weeks Series) HPV-2 11-12 years (+1-2 9 years (+4 weeks) 4 months 12 weeks (n) months) HPV-3(n) 11-12 years (+6 months) 9 years (+5 months) — — Influenza, inactivated(o) ≥6 months 6 months(p) 4 weeks 4 weeks IPV-1(e) 2 months 6 weeks 8 weeks 4 weeks IPV-2 4 months 10 weeks 8 weeks-14 months 4 weeks IPV-3 6-18 months 14 weeks 3-5 years 6 months IPV-4(q) 4-6 years 4 years — — LAIV(o) 2-49 years 2 years 4 weeks 4 weeks MenACWY-1(r) 11-12 years 2 months(s) 4-5 years 8 weeks MenACWY-2 16 years 11 years (+ 8 weeks)(t) — — MenB-1 Healthy adolescents: 16 -

HPV and Adolescent Vaccine Toolkit: Clinician Guide Contents
HPV and Adolescent Vaccine Toolkit: Clinician Guide CONTENTS I. 2018 IMMUNIZATION SCHEDULES & SCREENING RESOURCES Recommended & Catch-up Immunization Schedule (Birth-18 Years) Lists the ages or age range each vaccine is recommended. Schedules are updated annually. Please visit https://www.cdc.gov/vaccines/schedules/ for the most up-to-date schedules. Clinician FAQ: CDC Recommendations for HPV Vaccine 2-Dose Schedules Helps explain the new HPV vaccine recommendation for adolescents (2 doses recommended for adolescents starting the series before their 15th birthday; 3 doses recommended for adolescents starting the series after their 15th birthday) and provides tips for talking to parents about the change. HPV 2-Dose Decision Tree Follow the decision tree chart to determine whether your patient needs two or three doses of HPV vaccine. II. ADDRESSING VACCINE HESITANCY Talking to Parents About the HPV Vaccine A collection of questions parents may have surrounding the HPV vaccine and responses healthcare providers can use to address the concerns. Let’s Talk Vaccines: A Guide to Conversations About Immunizations Parents ask tough questions! Use this resource from Northwest Vax to provide a strong recommendation using the Ask. Acknowledge. Advise model. III. BEST PRACTICES AND STRATEGIES FOR IMPROVING IMMUNIZATION COVERAGE RATES Strategies for Improving Adolescent Immunization Coverage Rates Use the strategies in this AAP resource to help your practice improve adolescent immunization coverage rates among your patients. Documenting Parental Refusal to Have Their Children Vaccinated Provides tips from the AAP on ways to communicate with and educate parents who refuse immunizations. Includes a template for use by health care providers to document refusals. -

1 What Is the Efficacy of COVID-19 Vaccinations in Preventing Disease Transmission to the Non-Vaccinated?
National Health Library and Knowledge Service | Evidence Team CURRENT AS AT 02 April 2021 Summary of Evidence: COVID-19 | Question 199 VERSION 1.0 The following information resources have been selected by the National Health Library and Knowledge Service Evidence Virtual Team in response to a question from the National Immunisation Advisory Committee (NIAC). The resources are listed in our estimated order of relevance to practicing healthcare professionals confronted with this scenario in an Irish context. In respect of the evolving global situation and rapidly changing evidence base, it is advised to use hyperlinked sources in this document to ensure that the information you are disseminating to the public or applying in clinical practice is the most current, valid and accurate. For further information on the methodology used in the compilation of this document including a complete list of sources consulted please see our National Health Library and Knowledge Service Summary of Evidence Protocol. QUESTION 199 What is the efficacy of COVID-19 vaccinations in preventing disease transmission to the non-vaccinated? Question 199 was prepared by the National Health Library and Knowledge Service in collaboration with the Research Subgroup of the National Immunisation Advisory Committee (NIAC). National Health Library and NIAC Knowledge Service | Evidence Team 1 National Health Library and Knowledge Service | Evidence Team CURRENT AS AT 02 April 2021 Summary of Evidence: COVID-19 | Question 199 VERSION 1.0 What is the efficacy of COVID-19 vaccinations in preventing disease transmission to the non-vaccinated? Main Points 1. Emerging evidence suggests that COVID-19 vaccines may also reduce asymptomatic infection, and potentially transmission. -

Vaccination to Prevent COVID-19
Vaccination to prevent COVID-19 International Parkinson and Movement Disorder Society Scientific Issues Committee Alvaro Sanchez-Ferro, Lorraine Kalia, Roy Alcalay, Helen Chiang, Un Jung Kang, Stella Papa Emergency use authorization of COVID-19 vaccines • The U.S. Food & Drug Administration (FDA), the European Medicines Agency (EMA), and Health Canada among other agencies have recently approved BNT162b2 (Pfizer/BioNTech) and mRNA-1273 (Moderna) vaccines for emergency use authorization or regular authorization for one year in the case of EMA. • The approval process by these regulatory entities includes thorough analyses of completed Phase III data provided by vaccine developers in a transparent process that includes peer-reviewed publication of full data sets. • The approval of both vaccines met the high standards required for use authorization after complete data scrutiny and validation, as required in the normal process of a vaccine approval. • Additional COVID-19 vaccines that are still in experimental phases II and III will eventually undergo the same scrutiny before emergency use authorization. Questions and Concerns The development and approval of COVID-19 vaccines reached record times compared with the typical timeframes of previous vaccines. The regulatory agencies have also expedited the vaccines approval for emergency use authorization or limited authorization for one year. As a result of this accelerated pace, there are doubts and concerns about the benefits and risks of these new vaccines that need to be addressed. In this presentation we will discuss critical points about: • Properties of the novel mRNA-based vaccines, • Efficacy data, • Safety data, • Clinical significance particularly for patients with Parkinson’s disease (PD). -

TB Vaccine R&D Roadmap Background Document
TB Vaccine R&D Roadmap Background Document Version: 4.0 Date: 10.12.2020 This document summarizes the state-of-the-art in research and development for new vaccines for tuberculosis (TB). It is meant as a background document for a TB Vaccine Research & Development Roadmap and support the various consultations that are being held as part of the process of its development.a This background document seeks to provide an overview of the TB vaccine development goals, the current vaccine R&D pipeline, issues in clinical development, (new) directions in discovery and preclinical research, and considerations about moving vaccine candidates through the pipeline. It is meant as a living document, that will be updated as the Roadmap development process goes along. It is not meant to be exhaustive but to provide the reader with sufficient background to understand the Roadmap’s considerations and recommendations. For more detail the reader is referred to a number of recent reviews on the topic 1 2 3 4 5 6. The recommendations for TB vaccine R&D recently published by a number of stakeholders in have been added as Annex 17. LIST OF ABBREVIATIONS BCG Bacille Calmette-Guérin CHIM Controlled human infection model DS-TB Drug-susceptible tuberculosis IAVI International AIDS Vaccine Initiative IGRA Interferon-gamma release assay MDR-TB Multidrug-resistant tuberculosis MIP Mycobacterium indicus pranii Mtb Mycobacterium tuberculosis NHP Non-human primate PDP product Development Partnership PoD Prevention of Disease (clinical endpoint) PoI Prevention of Infection (clinical endpoint) PoR Prevention of Recurrence (clinical endpoint) PPC Preferred Product Characteristic R&D Research and development TB Tuberculosis TBVI Tuberculosis Vaccine Initiative TST Tuberculin skin test WHO World Health Organization a The TB Vaccine R&D Roadmap is being developed by the Amsterdam Institute for Global health and Development and the World Health organization, with financial support from the European & Developing Countries Clinical Trials Partnership (EDCTP).