Vaccine Information Table UPDATED FEBRUARY 28, 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

0 January to July 2021
0 www.journalsofindia.com January to July 2021 SCIENCE & TECH ............................................................................................................................................................... 6 1. REUSABLE LAUNCH VEHICLE TECHNOLOGY DEMONSTRATION PROGRAMME(RLV-TD) ................................................. 6 2. GAGANYAAN MISSION ..................................................................................................................................................... 6 3. MARS ORBITER MISSION (MOM) ..................................................................................................................................... 6 4. CHANDRAYAAN MISSION................................................................................................................................................. 7 5. SOLAR MISSION ............................................................................................................................................................... 8 6. ARTEMIS ACCORD ............................................................................................................................................................ 9 7. NATIONAL MISSION ON INTERDISCIPLINARY CYBER-PHYSICAL SYSTEM (NMICPS) ....................................................... 10 8. SMART ANTI-AIRFIELD WEAPON (SAAW) ...................................................................................................................... 10 9. AQUAPONICS ................................................................................................................................................................ -

Safety of Immunization During Pregnancy a Review of the Evidence
Safety of Immunization during Pregnancy A review of the evidence Global Advisory Committee on Vaccine Safety © World Health Organization 2014 All rights reserved. Publications of the World Health Organization are available on the WHO website (www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications –whether for sale or for non-commercial distribution– should be addressed to WHO Press through the WHO website (www.who.int/about/licensing/copyright_form/en/index.html). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. -

Vaccines for Preteens
| DISEASES and the VACCINES THAT PREVENT THEM | INFORMATION FOR PARENTS Vaccines for Preteens: What Parents Should Know Last updated JANUARY 2017 Why does my child need vaccines now? to get vaccinated. The best time to get the flu vaccine is as soon as it’s available in your community, ideally by October. Vaccines aren’t just for babies. Some of the vaccines that While it’s best to be vaccinated before flu begins causing babies get can wear off as kids get older. And as kids grow up illness in your community, flu vaccination can be beneficial as they may come in contact with different diseases than when long as flu viruses are circulating, even in January or later. they were babies. There are vaccines that can help protect your preteen or teen from these other illnesses. When should my child be vaccinated? What vaccines does my child need? A good time to get these vaccines is during a yearly health Tdap Vaccine checkup. Your preteen or teen can also get these vaccines at This vaccine helps protect against three serious diseases: a physical exam required for sports, school, or camp. It’s a tetanus, diphtheria, and pertussis (whooping cough). good idea to ask the doctor or nurse every year if there are any Preteens should get Tdap at age 11 or 12. If your teen didn’t vaccines that your child may need. get a Tdap shot as a preteen, ask their doctor or nurse about getting the shot now. What else should I know about these vaccines? These vaccines have all been studied very carefully and are Meningococcal Vaccine safe. -

Meningococcal Vaccine Q & a for Healthcare Providers
Meningococcal Vaccine Q & A for Healthcare Providers School meningococcal vaccine requirements Q1: When did the school meningococcal vaccine requirement take effect? A1: The meningococcal vaccine school requirement took effect on September 1, 2016. Q2: For what grades is meningococcal vaccine required? A2: Meningococcal vaccine is currently required for students entering or attending grades 7 through 12 in public, private and parochial New York State (NYS) schools. Q3: How many doses of meningococcal vaccine are required for grades 7 through 11? A3: One dose of meningococcal conjugate vaccine (MenACWY; sometimes abbreviated as MCV4; brand names Menactra or Menveo) is required for entry into grades 7 through 11. Q4: How many doses of meningococcal vaccine are required for grade 12? A4: A total of two doses of MenACWY vaccine, administered a minimum of 8 weeks apart, are required for entry into grade 12. The second dose must be administered no sooner than 16 years of age. However, if the first dose of MenACWY vaccine was received at 16 years of age or older, then a second dose will not be required. The NYS school immunization requirements allow for a grace period of up to 4 days before the 16th birthday for receipt of the dose. A dose of vaccine received 5 or more days before the 16th birthday will not meet the 12th grade meningococcal vaccine requirement. Q5: Is serogroup B meningococcal vaccine (MenB vaccine) required for grade 12? A5: No, MenB vaccine is not required for school attendance in NYS. In addition, doses of MenB vaccine will not meet the NYS MenACWY vaccine requirement. -

PROOF of IMMUNIZATION COMPLIANCE NORTHWESTERN STATE UNIVERSITY of LOUISIANA (Louisiana R.S
PROOF OF IMMUNIZATION COMPLIANCE NORTHWESTERN STATE UNIVERSITY OF LOUISIANA (Louisiana R.S. 17:170.1 Schools of Higher Learning) SS Number: _____________________________________________ Date of Birth: Month _________________ Date ___________________ Year ________________ Name: __________________________________________________________________________________________________________________________________ Please Print (Last) (First) (Middle) Address: ________________________________________________________________________________________________________________________________ City: ______________________________________________________ State: ________________________________ ZIP Code: _____________________________ UNIVERSITY REQUIRED IMMUNIZATIONS: Physician or Other Health Care Provider Verification: (See other side) M-M-R (Measles, Mumps, Rubella-2 Doses Required) Tetanus Diphtheria (Td) Pertussis (Tdap) OR First dose: ___________________ Serologic Test: __________________ Td: ___________________ (Date) (Date within 10 years) (Date) OR Second dose: __________________ (Date) Result: _________________________ (Date) Tdap: ___________________ (Date within 10 years) OR □ Born before 1956 Meningitis Vaccine ACYW-135 (TWO doses of meningococcal conjugate vaccination separated by at least eight weeks.) First dose: ____________________________________ Vaccine Type: _______________________________________ (Date) Second dose: __________________________________ Vaccine Type: _______________________________________ (Date) UNIVERSITY REQUIRED IMMUNIZATIONS: -

Journal of Vaccines & Vaccination
ccines & a V f V a o c l c i a n n a r t u i o o n J Ching, et al., J Vaccines Vaccin 2014, 5:6 Journal of Vaccines & Vaccination DOI: 10.4172/2157-7560.1000257 ISSN: 2157-7560 Research Article Open Access Evaluation of a Recombinant Vaccine Candidate r56Lc-1 in a Chigger Challenge Mouse Model Wei-Mei Ching1,3*, Woradee Lurchachaiwong2, Zhiwen Zhang1,3 Temitayo Awoyomi1,3, Chien-Chung Chao1,3 and Anthony Schuster2 1Viral and Rickettsial Diseases Department, Infectious Diseases Directorate, Naval Medical Research Center, Silver Spring, USA 2Entomology Department, Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand 3Uniformed Services University of the Health Sciences, Bethesda, USA *Corresponding author: Wei-Mei Ching, PhD, Viral and Rickettsial Diseases Department, Infectious Diseases Directorate, Naval Medical Research Center, 503 Robert Grant Ave, RM3N71, Silver Spring, MD 20910, USA, Tel: 301 319 7438; Fax: 301 319 7451; E-mail: [email protected] Received date: 15 Sep 2014; Accepted date: 24 Oct 2014; Published date: 27 Oct 2014 Copyright: © 2014 Ching WM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Scrub typhus, an acute, febrile disease is transmitted by the bite of an Orientia infected chigger. We evaluated the protective potential of a recombinant 56 kDa antigen in a chigger challenge mouse model which mimics the natural transmission of Orientia. Chiggers from an L. -

Vaccination and Anaphylaxis
14 FORENSIC SCIENCE Croat Med J. 2017;58:14-25 https://doi.org/10.3325/cmj.2017.58.14 Vaccination and anaphylaxis: a Cristian Palmiere1, Camilla Tettamanti2, Maria Pia forensic perspective Scarpelli1 1CURML, University Center of Legal Medicine, Lausanne 25, Switzerland 2Department of Legal Medicine, Aim To review the available literature pertaining to fatali- University of Genova, Genova, Italy ties following vaccine administration and, in particular, cas- es of vaccine-related fatal anaphylaxis. Method The MEDLINE database was systematically searched up to March 2016 to identify all relevant articles pertaining to fatal cases of anaphylaxis following vaccine administration. Results Six papers pertaining to fatal anaphylaxis following vaccination were found relevant. Mast cell tryptase and to- tal IgE concentration was assessed exclusively in one case. Laryngeal edema was not detected in any of these cases, whereas eosinophil or mast cell infiltration was observed in lymphoid organs. In one case, immunohistochemical in- vestigations using anti-tryptase antibodies allowed pulmo- nary mast cells and degranulating mast cells with tryptase- positive material outside to be identified. Conclusion In any suspected IgE-mediated fatal anaphy- lactic cases, biochemical investigations should be system- atically performed for forensic purposes. Splenic tissue should be routinely sampled for immunohistochemical investigations in all suspected anaphylaxis-related deaths and mast cell/eosinophil infiltrations should be systemati- cally sought out in -

COVID-19 Vaccines: Summary of Current State-Of-Play Prepared Under Urgency 21 May 2020 – Updated 16 July 2020
Office of the Prime Minister’s Chief Science Advisor Kaitohutohu Mātanga Pūtaiao Matua ki te Pirimia COVID-19 vaccines: Summary of current state-of-play Prepared under urgency 21 May 2020 – updated 16 July 2020 The COVID-19 pandemic has spurred a global effort to find a vaccine to protect people from SARS- CoV-2 infection. This summary highlights selected candidates, explains the different types of vaccines being investigated and outlines some of the potential issues and risks that may arise during the clinical testing process and beyond. Key points • There are at least 22 vaccine candidates registered in clinical (human) trials, out of a total of at least 194 in various stages of active development. • It is too early to choose a particular frontrunner as we lack safety and efficacy information for these candidates. • It is difficult to predict when a vaccine will be widely available. The fastest turnaround from exploratory research to vaccine approval was previously 4–5 years (ebolavirus vaccine), although it is likely that current efforts will break this record. • There are a number of challenges associated with accelerated vaccine development, including ensuring safety, proving efficacy in a rapidly changing pandemic landscape, and scaling up manufacture. • The vaccine that is licensed first will not necessarily confer full or long-lasting protection. 1 Contents Key points .................................................................................................................................. 1 1. Types of vaccines ............................................................................................................... -
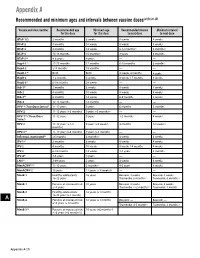
Recommended and Minimum Ages and Intervals Between Doses
Appendix A Recommended and minimum ages and intervals between vaccine doses(a),(b),(c),(d) Vaccine and dose number Recommended age Minimum age Recommended interval Minimum interval for this dose for this dose to next dose to next dose DTaP-1(e) 2 months 6 weeks 8 weeks 4 weeks DTaP-2 4 months 10 weeks 8 weeks 4 weeks DTaP-3 6 months 14 weeks 6-12 months(f) 6 months(f) DTaP-4 15-18 months 15 months(f) 3 years 6 months DTaP-5(g) 4-6 years 4 years — — HepA-1(e) 12-23 months 12 months 6-18 months 6 months HepA-2 ≥18 months 18 months — — HepB-1(h) Birth Birth 4 weeks-4 months 4 weeks HepB-2 1-2 months 4 weeks 8 weeks-17 months 8 weeks HepB-3(i) 6-18 months 24 weeks — — Hib-1(j) 2 months 6 weeks 8 weeks 4 weeks Hib-2 4 months 10 weeks 8 weeks 4 weeks Hib-3(k) 6 months 14 weeks 6-9 months 8 weeks Hib-4 12-15 months 12 months — — HPV-1 (Two-Dose Series)(l) 11-12 years 9 years 6 months 5 months HPV-2 11-12 years (+6 months) 9 years +5 months(m) — — HPV-1(n) (Three-Dose 11-12 years 9 years 1-2 months 4 weeks Series) HPV-2 11-12 years (+1-2 9 years (+4 weeks) 4 months 12 weeks (n) months) HPV-3(n) 11-12 years (+6 months) 9 years (+5 months) — — Influenza, inactivated(o) ≥6 months 6 months(p) 4 weeks 4 weeks IPV-1(e) 2 months 6 weeks 8 weeks 4 weeks IPV-2 4 months 10 weeks 8 weeks-14 months 4 weeks IPV-3 6-18 months 14 weeks 3-5 years 6 months IPV-4(q) 4-6 years 4 years — — LAIV(o) 2-49 years 2 years 4 weeks 4 weeks MenACWY-1(r) 11-12 years 2 months(s) 4-5 years 8 weeks MenACWY-2 16 years 11 years (+ 8 weeks)(t) — — MenB-1 Healthy adolescents: 16 -

India: the World's Pharmacy Expands Its Reach in Global Health
New York | New Delhi | Rio de Janeiro Nairobi | Johannesburg | London India: The World’s Pharmacy Expands Its Reach in Global Health March 2021 This white paper was last updated on 1 March 2021 Global Health Strategies 18/1, 2nd Floor Shaheed Bhawan, Aruna Asaf Ali Marg, New Delhi, 110 067 www.globalhealthstrategies.com Twitter: @GHS Contents Executive Summary 02 India’s response to COVID-19 03 ∙ Pharmaceuticals and Biosimilars 04 ∙ Vaccines 05 ∙ Diagnostics 06 Evolution of India’s pharmaceutical industry 07 ∙ Milestones 08 Case Studies ∙ Hepatitis B Vaccine 10 ∙ Anti-retroviral Drugs 11 ∙ MenAfriVac 12 ∙ Complex Generics 13 ∙ Insulin 14 ∙ Monoclonal Antibodies 15 ∙ Vaccine for Rotavirus 16 ∙ Typhoid Conjugate Vaccine 17 Conclusion: Looking Forward 18 Executive Summary India’s pharmaceutical industry is already playing a pivotal role in the scale-up of pharmaceuticals and diagnostics to combat the global COVID-19 pandemic. It is poised to play an even more dominant role as biological products – preventive vaccines and cutting-edge biotechnology such as monoclonal antibodies– come to the fore. Even before the pandemic, Indian manufacturers produced vast quantities of generic antiviral drugs that turned HIV from a death sentence to a chronic manageable condition in developing countries. India’s global dominance in generic drugs and vaccine manufacturing has earned it the label “Pharmacy of the World”. COVID-19 only strengthens the case for this moniker. So far, India has supplied medicines to 133 countries to fight the pandemic. Six Indian manufacturers have been granted royalty-free licenses by Gilead to manufacture the first antiviral drug approved by the U.S. -

Artículos Científicos
Editor: NOEL GONZÁLEZ GOTERA Número 064 Diseño: Lic. Roberto Chávez y Liuder Machado. Semana 291212 - 040113 Foto: Lic. Belkis Romeu e Instituto Finlay La Habana, Cuba. ARTÍCULOS CIENTÍFICOS P ublicaciones incluidas en P ubMED durante el período comprendido entre el 29 de diciembre de 2012 y el 4 de enero de 2013. Total de artículos reuperados con “vaccin*” en título: 68 Vacunas meningococo (Neisseria meningitidis) 13. Up take of meningococcal va ccine in Arizona schoolchildren after implementation of school- entry immunization requirements. Simpson JE, Hills RA, Allwes D, Rasmussen L. Public Health Rep. 2013 Jan;128(1):37-45. PMID: 23277658 [PubMed - in process] Related citations 33. A cute Cerebellar Ataxia Following Meningococcal Group C Conjugate V accination. Cutroneo PM, Italiano D, Trifirò G, Tortorella G, Russo A, Isola S, Caputi AP, Spina E. J Child Neurol. 2012 Dec 28. [Epub ahead of print] PMID: 23275434 [PubMed - as supplied by publisher] Related citations 39. Preclinical safety and immunogenicity evaluation of a nonavalent PorA native outer m embrane vesicle va ccine against serogroup B meningococcal disease. Kaaijk P, van Straaten I, van de Waterbeemd B, Boot EP, Levels LM, van Dijken HH, van den Dobbelsteen GP. 1 Vaccine. 2012 Dec 27. doi:pii: S0264-410X(12)01815-4. 10.1016/j.vaccine.2012.12.031. [Epub ahead of print] PMID: 23273968 [PubMed - as supplied by publisher] Related citations 40. P riorities for research on meningocccal disease and the impact of serogroup A va ccination in the African meningitis belt. [No authors listed] Vaccine. 2012 Dec 27. doi:pii: S0264-410X(12)01820-8. -

HPV and Adolescent Vaccine Toolkit: Clinician Guide Contents
HPV and Adolescent Vaccine Toolkit: Clinician Guide CONTENTS I. 2018 IMMUNIZATION SCHEDULES & SCREENING RESOURCES Recommended & Catch-up Immunization Schedule (Birth-18 Years) Lists the ages or age range each vaccine is recommended. Schedules are updated annually. Please visit https://www.cdc.gov/vaccines/schedules/ for the most up-to-date schedules. Clinician FAQ: CDC Recommendations for HPV Vaccine 2-Dose Schedules Helps explain the new HPV vaccine recommendation for adolescents (2 doses recommended for adolescents starting the series before their 15th birthday; 3 doses recommended for adolescents starting the series after their 15th birthday) and provides tips for talking to parents about the change. HPV 2-Dose Decision Tree Follow the decision tree chart to determine whether your patient needs two or three doses of HPV vaccine. II. ADDRESSING VACCINE HESITANCY Talking to Parents About the HPV Vaccine A collection of questions parents may have surrounding the HPV vaccine and responses healthcare providers can use to address the concerns. Let’s Talk Vaccines: A Guide to Conversations About Immunizations Parents ask tough questions! Use this resource from Northwest Vax to provide a strong recommendation using the Ask. Acknowledge. Advise model. III. BEST PRACTICES AND STRATEGIES FOR IMPROVING IMMUNIZATION COVERAGE RATES Strategies for Improving Adolescent Immunization Coverage Rates Use the strategies in this AAP resource to help your practice improve adolescent immunization coverage rates among your patients. Documenting Parental Refusal to Have Their Children Vaccinated Provides tips from the AAP on ways to communicate with and educate parents who refuse immunizations. Includes a template for use by health care providers to document refusals.