Molecular Changes in Tissue Proteome During Prostate Cancer Development: Proof-Of-Principle Investigation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Histone Isoform H2A1H Promotes Attainment of Distinct Physiological
Bhattacharya et al. Epigenetics & Chromatin (2017) 10:48 DOI 10.1186/s13072-017-0155-z Epigenetics & Chromatin RESEARCH Open Access Histone isoform H2A1H promotes attainment of distinct physiological states by altering chromatin dynamics Saikat Bhattacharya1,4,6, Divya Reddy1,4, Vinod Jani5†, Nikhil Gadewal3†, Sanket Shah1,4, Raja Reddy2,4, Kakoli Bose2,4, Uddhavesh Sonavane5, Rajendra Joshi5 and Sanjay Gupta1,4* Abstract Background: The distinct functional efects of the replication-dependent histone H2A isoforms have been dem- onstrated; however, the mechanistic basis of the non-redundancy remains unclear. Here, we have investigated the specifc functional contribution of the histone H2A isoform H2A1H, which difers from another isoform H2A2A3 in the identity of only three amino acids. Results: H2A1H exhibits varied expression levels in diferent normal tissues and human cancer cell lines (H2A1C in humans). It also promotes cell proliferation in a context-dependent manner when exogenously overexpressed. To uncover the molecular basis of the non-redundancy, equilibrium unfolding of recombinant H2A1H-H2B dimer was performed. We found that the M51L alteration at the H2A–H2B dimer interface decreases the temperature of melting of H2A1H-H2B by ~ 3 °C as compared to the H2A2A3-H2B dimer. This diference in the dimer stability is also refected in the chromatin dynamics as H2A1H-containing nucleosomes are more stable owing to M51L and K99R substitu- tions. Molecular dynamic simulations suggest that these substitutions increase the number of hydrogen bonds and hydrophobic interactions of H2A1H, enabling it to form more stable nucleosomes. Conclusion: We show that the M51L and K99R substitutions, besides altering the stability of histone–histone and histone–DNA complexes, have the most prominent efect on cell proliferation, suggesting that the nucleosome sta- bility is intimately linked with the physiological efects observed. -

Analysis of Trans Esnps Infers Regulatory Network Architecture
Analysis of trans eSNPs infers regulatory network architecture Anat Kreimer Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2014 © 2014 Anat Kreimer All rights reserved ABSTRACT Analysis of trans eSNPs infers regulatory network architecture Anat Kreimer eSNPs are genetic variants associated with transcript expression levels. The characteristics of such variants highlight their importance and present a unique opportunity for studying gene regulation. eSNPs affect most genes and their cell type specificity can shed light on different processes that are activated in each cell. They can identify functional variants by connecting SNPs that are implicated in disease to a molecular mechanism. Examining eSNPs that are associated with distal genes can provide insights regarding the inference of regulatory networks but also presents challenges due to the high statistical burden of multiple testing. Such association studies allow: simultaneous investigation of many gene expression phenotypes without assuming any prior knowledge and identification of unknown regulators of gene expression while uncovering directionality. This thesis will focus on such distal eSNPs to map regulatory interactions between different loci and expose the architecture of the regulatory network defined by such interactions. We develop novel computational approaches and apply them to genetics-genomics data in human. We go beyond pairwise interactions to define network motifs, including regulatory modules and bi-fan structures, showing them to be prevalent in real data and exposing distinct attributes of such arrangements. We project eSNP associations onto a protein-protein interaction network to expose topological properties of eSNPs and their targets and highlight different modes of distal regulation. -
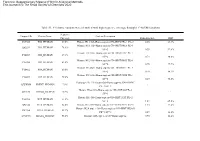
Table S1. 49 Histone Variants Were Identified with High Sequence Coverage Through LC-MS/MS Analysis Electronic Supplementary
Electronic Supplementary Material (ESI) for Analytical Methods. This journal is © The Royal Society of Chemistry 2020 Table S1. 49 histone variants were identified with high sequence coverage through LC-MS/MS analysis Sequence Uniprot IDs Protein Name Protein Description Coverage Ratio E2+/E2- RSD P07305 H10_HUMAN 67.5% Histone H1.0 OS=Homo sapiens GN=H1F0 PE=1 SV=3 4.85 23.3% Histone H1.1 OS=Homo sapiens GN=HIST1H1A PE=1 Q02539 H11_HUMAN 74.4% SV=3 0.35 92.6% Histone H1.2 OS=Homo sapiens GN=HIST1H1C PE=1 P16403 H12_HUMAN 67.1% SV=2 0.73 80.6% Histone H1.3 OS=Homo sapiens GN=HIST1H1D PE=1 P16402 H13_HUMAN 63.8% SV=2 0.75 77.7% Histone H1.4 OS=Homo sapiens GN=HIST1H1E PE=1 P10412 H14_HUMAN 69.0% SV=2 0.70 80.3% Histone H1.5 OS=Homo sapiens GN=HIST1H1B PE=1 P16401 H15_HUMAN 79.6% SV=3 0.29 98.3% Testis-specific H1 histone OS=Homo sapiens GN=H1FNT Q75WM6 H1FNT_HUMAN 7.8% \ \ PE=2 SV=3 Histone H1oo OS=Homo sapiens GN=H1FOO PE=2 Q8IZA3 H1FOO_HUMAN 5.2% \ \ SV=1 Histone H1t OS=Homo sapiens GN=HIST1H1T PE=2 P22492 H1T_HUMAN 31.4% SV=4 1.42 65.0% Q92522 H1X_HUMAN 82.6% Histone H1x OS=Homo sapiens GN=H1FX PE=1 SV=1 1.15 33.2% Histone H2A type 1 OS=Homo sapiens GN=HIST1H2AG P0C0S8 H2A1_HUMAN 99.2% PE=1 SV=2 0.57 26.8% Q96QV6 H2A1A_HUMAN 58.0% Histone H2A type 1-A OS=Homo sapiens 0.90 11.2% GN=HIST1H2AA PE=1 SV=3 Histone H2A type 1-B/E OS=Homo sapiens P04908 H2A1B_HUMAN 99.2% GN=HIST1H2AB PE=1 SV=2 0.92 30.2% Histone H2A type 1-C OS=Homo sapiens Q93077 H2A1C_HUMAN 100.0% GN=HIST1H2AC PE=1 SV=3 0.76 27.6% Histone H2A type 1-D OS=Homo sapiens P20671 -

Environmental Influences on Endothelial Gene Expression
ENDOTHELIAL CELL GENE EXPRESSION John Matthew Jeff Herbert Supervisors: Prof. Roy Bicknell and Dr. Victoria Heath PhD thesis University of Birmingham August 2012 University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. ABSTRACT Tumour angiogenesis is a vital process in the pathology of tumour development and metastasis. Targeting markers of tumour endothelium provide a means of targeted destruction of a tumours oxygen and nutrient supply via destruction of tumour vasculature, which in turn ultimately leads to beneficial consequences to patients. Although current anti -angiogenic and vascular targeting strategies help patients, more potently in combination with chemo therapy, there is still a need for more tumour endothelial marker discoveries as current treatments have cardiovascular and other side effects. For the first time, the analyses of in-vivo biotinylation of an embryonic system is performed to obtain putative vascular targets. Also for the first time, deep sequencing is applied to freshly isolated tumour and normal endothelial cells from lung, colon and bladder tissues for the identification of pan-vascular-targets. Integration of the proteomic, deep sequencing, public cDNA libraries and microarrays, delivers 5,892 putative vascular targets to the science community. -

Smith Bacterial SBP56 Identified As a Cu-Dependent Methanethiol
Bacterial SBP56 identified as a Cu-dependent methanethiol oxidase widely distributed in the biosphere EYICE, Özge, MYRONOVA, Nataliia, POL, Arjan, CARRIÓN, Ornella, TODD, Jonathan D, SMITH, Thomas <http://orcid.org/0000-0002-4246-5020>, GURMAN, Stephen J, CUTHBERTSON, Adam, MAZARD, Sophie, MENNINK-KERSTEN, Monique Ash, BUGG, Timothy Dh, ANDERSSON, Karl Kristoffer, JOHNSTON, Andrew Wb, OP DEN CAMP, Huub Jm and SCHÄFER, Hendrik Available from Sheffield Hallam University Research Archive (SHURA) at: http://shura.shu.ac.uk/17252/ This document is the author deposited version. You are advised to consult the publisher's version if you wish to cite from it. Published version EYICE, Özge, MYRONOVA, Nataliia, POL, Arjan, CARRIÓN, Ornella, TODD, Jonathan D, SMITH, Thomas, GURMAN, Stephen J, CUTHBERTSON, Adam, MAZARD, Sophie, MENNINK-KERSTEN, Monique Ash, BUGG, Timothy Dh, ANDERSSON, Karl Kristoffer, JOHNSTON, Andrew Wb, OP DEN CAMP, Huub Jm and SCHÄFER, Hendrik (2018). Bacterial SBP56 identified as a Cu-dependent methanethiol oxidase widely distributed in the biosphere. The ISME journal, 1 (12), 145-160. Copyright and re-use policy See http://shura.shu.ac.uk/information.html Sheffield Hallam University Research Archive http://shura.shu.ac.uk OPEN The ISME Journal (2017), 1–16 www.nature.com/ismej ORIGINAL ARTICLE Bacterial SBP56 identified as a Cu-dependent methanethiol oxidase widely distributed in the biosphere Özge Eyice1,2,9, Nataliia Myronova1,9, Arjan Pol3, Ornella Carrión4, Jonathan D Todd4, Tom J Smith5, Stephen J Gurman6, Adam Cuthbertson1, -

Genome-Wide Screen of Cell-Cycle Regulators in Normal and Tumor Cells
bioRxiv preprint doi: https://doi.org/10.1101/060350; this version posted June 23, 2016. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Genome-wide screen of cell-cycle regulators in normal and tumor cells identifies a differential response to nucleosome depletion Maria Sokolova1, Mikko Turunen1, Oliver Mortusewicz3, Teemu Kivioja1, Patrick Herr3, Anna Vähärautio1, Mikael Björklund1, Minna Taipale2, Thomas Helleday3 and Jussi Taipale1,2,* 1Genome-Scale Biology Program, P.O. Box 63, FI-00014 University of Helsinki, Finland. 2Science for Life laboratory, Department of Biosciences and Nutrition, Karolinska Institutet, SE- 141 83 Stockholm, Sweden. 3Science for Life laboratory, Division of Translational Medicine and Chemical Biology, Department of Medical Biochemistry and Biophysics, Karolinska Institutet, S-171 21 Stockholm, Sweden To identify cell cycle regulators that enable cancer cells to replicate DNA and divide in an unrestricted manner, we performed a parallel genome-wide RNAi screen in normal and cancer cell lines. In addition to many shared regulators, we found that tumor and normal cells are differentially sensitive to loss of the histone genes transcriptional regulator CASP8AP2. In cancer cells, loss of CASP8AP2 leads to a failure to synthesize sufficient amount of histones in the S-phase of the cell cycle, resulting in slowing of individual replication forks. Despite this, DNA replication fails to arrest, and tumor cells progress in an elongated S-phase that lasts several days, finally resulting in death of most of the affected cells. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Protein Targets of Acetaminophen Covalent Binding in Rat and Mouse
ORIGINAL RESEARCH published: XX XX 2021 doi: 10.3389/fchem.2021.736788 1 58 2 59 3 60 4 61 5 62 6 63 7 64 8 65 9 66 10 Protein Targets of Acetaminophen 67 11 68 12 Covalent Binding in Rat and Mouse 69 13 70 14 Q2 Liver Studied by LC-MS/MS 71 15 Q3 72 Q1 16 Timon Geib, Ghazaleh Moghaddam, Aimee Supinski, Makan Golizeh† and Lekha Sleno* Q4 73 17 Q5 74 18 Chemistry Department, Université du Québec à Montréal, Montréal, QC, Canada Q6 75 19 76 20 Acetaminophen (APAP) is a mild analgesic and antipyretic used commonly worldwide. 77 21 78 Although considered a safe and effective over-the-counter medication, it is also the leading 22 79 23 cause of drug-induced acute liver failure. Its hepatotoxicity has been linked to the covalent 80 24 binding of its reactive metabolite, N-acetyl p-benzoquinone imine (NAPQI), to proteins. The 81 Edited by: 25 aim of this study was to identify APAP-protein targets in both rat and mouse liver, and to 82 26 Marcus S Cooke, 83 University of South Florida, compare the results from both species, using bottom-up proteomics with data-dependent 27 United States 84 28 high resolution mass spectrometry and targeted multiple reaction monitoring (MRM) 85 Reviewed by: 29 experiments. Livers from rats and mice, treated with APAP, were homogenized and 86 Hartmut Jaeschke, 30 University of Kansas Medical Center digested by trypsin. Digests were then fractionated by mixed-mode solid-phase extraction 87 31 Research Institute, United States prior to liquid chromatography-tandem mass spectrometry (LC-MS/MS). -

Volatile Sulfur Compounds in Coastal Acid Sulfate Soils, Northern N.S.W
VOLATILE SULFUR COMPOUNDS IN COASTAL ACID SULFATE SOILS, NORTHERN N.S.W Andrew Stephen Kinsela A thesis submitted in fulfilment of the requirements for the degree of Doctor of Philosophy School of Biological, Earth & Environmental Sciences THE UNIVERSITY OF NEW SOUTH WALES, AUSTRALIA 2007 DECLARATION ORIGINALITY STATEMENT ‘I hereby declare that this submission is my own work and to the best of my knowledge it contains no materials previously published or written by another person, or substantial proportions of material which have been accepted for the award of any other degree or diploma at UNSW or any other educational institution, except where due acknowledgement is made in the thesis. Any contribution made to the research by others, with whom I have worked at UNSW or elsewhere, is explicitly acknowledged in the thesis. I also declare that the intellectual content of this thesis is the product of my own work, except to the extent that assistance from others in the project's design and conception or in style, presentation and linguistic expression is acknowledged.’ Signed ………………………………………………… Date …………………………………………………… iii ACKNOWLEDGEMENTS There are numerous people who have assisted me throughout the course of my thesis. I therefore want to take this opportunity to thank a few of those who contributed appreciably, both directly and indirectly. First of all, I would like to express my heartfelt gratitude to my supervisor, Associate Professor Mike Melville. Mike’s initial teachings as part of my undergraduate studies first sparked my interest in soils. Since then his continued enthusiasm on the subject has helped shape the way I approach my own work. -

Supplementary Table 1
Supplementary Table 1. 492 genes are unique to 0 h post-heat timepoint. The name, p-value, fold change, location and family of each gene are indicated. Genes were filtered for an absolute value log2 ration 1.5 and a significance value of p ≤ 0.05. Symbol p-value Log Gene Name Location Family Ratio ABCA13 1.87E-02 3.292 ATP-binding cassette, sub-family unknown transporter A (ABC1), member 13 ABCB1 1.93E-02 −1.819 ATP-binding cassette, sub-family Plasma transporter B (MDR/TAP), member 1 Membrane ABCC3 2.83E-02 2.016 ATP-binding cassette, sub-family Plasma transporter C (CFTR/MRP), member 3 Membrane ABHD6 7.79E-03 −2.717 abhydrolase domain containing 6 Cytoplasm enzyme ACAT1 4.10E-02 3.009 acetyl-CoA acetyltransferase 1 Cytoplasm enzyme ACBD4 2.66E-03 1.722 acyl-CoA binding domain unknown other containing 4 ACSL5 1.86E-02 −2.876 acyl-CoA synthetase long-chain Cytoplasm enzyme family member 5 ADAM23 3.33E-02 −3.008 ADAM metallopeptidase domain Plasma peptidase 23 Membrane ADAM29 5.58E-03 3.463 ADAM metallopeptidase domain Plasma peptidase 29 Membrane ADAMTS17 2.67E-04 3.051 ADAM metallopeptidase with Extracellular other thrombospondin type 1 motif, 17 Space ADCYAP1R1 1.20E-02 1.848 adenylate cyclase activating Plasma G-protein polypeptide 1 (pituitary) receptor Membrane coupled type I receptor ADH6 (includes 4.02E-02 −1.845 alcohol dehydrogenase 6 (class Cytoplasm enzyme EG:130) V) AHSA2 1.54E-04 −1.6 AHA1, activator of heat shock unknown other 90kDa protein ATPase homolog 2 (yeast) AK5 3.32E-02 1.658 adenylate kinase 5 Cytoplasm kinase AK7 -

Dynamic Changes in the Urine Proteome in Two Ovarian Cancer Rat
bioRxiv preprint doi: https://doi.org/10.1101/604850; this version posted April 10, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Dynamic changes in the urine proteome in two ovarian cancer rat models Yuqiu Li 1,2, Linpei Zhang1,2, Wenshu Meng 1,2, Youhe Gao *1,2 1. Department of Biochemistry and Molecular Biology, Beijing Normal University, Beijing 100875; 2. Gene Engineering Drug and Biotechnology Beijing Key Laboratory, Beijing 100875 *Corresponding author: Youhe Gao Email: [email protected] Phone: 86-10-5880-4382; Fax: 86-10-6521-2284 Abstract: Ovarian cancer is the most lethal gynecological malignancy in women, and it is likely to metastasize and has a poor prognosis. The early and reliable diagnosis and monitoring of ovarian cancer is very important. Without a homeostasis mechanism, urine can reflect early systemic changes in the body and has a great potential to be used for the early detection of cancer. This study tested whether early changes could be detected in two ovarian cancer rat models. Two rat models were established by either intraperitoneal (i.p.) or orthotopic (o.t.) injection of NuTu-19 ovarian cancer cells in female Fischer344 rats. Urine samples from ovarian cancer rats were collected at five time points during cancer development, and urinary proteins from the rats were profiled by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS). Compared with pre-injection samples, 49 differential proteins that have human orthologues were significantly changed in the orthotopically injected model. -

Severe Child Form of Primary Hyperoxaluria Type 2
Konkoľová et al. BMC Medical Genetics (2017) 18:59 DOI 10.1186/s12881-017-0421-8 CASE REPORT Open Access Severe child form of primary hyperoxaluria type 2 - a case report revealing consequence of GRHPR deficiency on metabolism Jana Konkoľová1,2*, Ján Chandoga1,2, Juraj Kováčik3, Marcel Repiský2, Veronika Kramarová2, Ivana Paučinová3 and Daniel Böhmer1,2 Abstract Background: Primary hyperoxaluria type 2 is a rare monogenic disorder inherited in an autosomal recessive pattern. It results from the absence of the enzyme glyoxylate reductase/hydroxypyruvate reductase (GRHPR). As a consequence of deficient enzyme activity, excessive amounts of oxalate and L-glycerate are excreted in the urine, and are a source for the formation of calcium oxalate stones that result in recurrent nephrolithiasis and less frequently nephrocalcinosis. Case presentation: We report a case of a 10-month-old patient diagnosed with urolithiasis. Screening of inborn errors of metabolism, including the performance of GC/MS urine organic acid profiling and HPLC amino acid profiling, showed abnormalities, which suggested deficiency of GRHPR enzyme. Additional metabolic disturbances observed in the patient led us to seek other genetic determinants and the elucidation of these findings. Besides the elevated excretion of 3-OH-butyrate, adipic acid, which are typical marks of ketosis, other metabolites such as 3- aminoisobutyric acid, 3-hydroxyisobutyric acid, 3-hydroxypropionic acid and 2-ethyl-3-hydroxypropionic acids were observed in increased amounts in the urine. Direct sequencing of the GRHPR gene revealed novel mutation, described for the first time in this article c.454dup (p.Thr152Asnfs*39) in homozygous form. The frequent nucleotide variants were found in AGXT2 gene.