Capacitor 1 Farad P6-8012
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Realization of the Ohm and Farad Using the NBS Calculable Capacitor
IEEE TRANSACTIONS ON INSTRUMENTATION AND MEASUREMENT, VOL. 38, NO. 2, APRIL 1989 249 New Realization of the Ohm and Farad Using the NBS Calculable Capacitor JOHN Q. SHIELDS, RONALD F. DZIUBA, MEMBER, IEEE,AND HOWARD P. LAYER Abstrucl-Results of a new realization of the ohm and farad using part of the NBS effort. This paper describes our measure- the NBS calculable capacitor and associated apparatus are reported. ments and gives our latest results. The results show that both the NBS representation of the ohm and the NBS representation of the farad are changing with time, cNBSat the rate of -0.054 ppmlyear and FNssat the rate of 0.010 ppm/year. The 11. AC MEASUREMENTS realization of the ohm is of particular significance at this time because The measurement sequence used in the 1974 ohm and of its role in assigning an SI value to the quantized Hall resistance. The farad determinations [2] has been retained in the present estimated uncertainty of the ohm realization is 0.022 ppm (lo) while the estimated uncertainty of the farad realization is 0.014 ppm (la). NBS measurements. A 0.5-pF calculable cross-capacitor is used to measure a transportable 10-pF reference capac- itor which is carried to the laboratory containing the NBS I. INTRODUCTION bank of 10-pF fused silica reference capacitors. A 10: 1 HE NBS REPRESENTATION of the ohm is bridge is used in two stages to measure two 1000-pF ca- T based on the mean resistance of five Thomas-type pacitors which are in turn used as two arms of a special wire-wound resistors maintained in a 25°C oil bath at NBS frequency-dependent bridge for measuring two 100-kQ Gaithersburg, MD. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

The ST System of Units Leading the Way to Unification
The ST system of units Leading the way to unification © Xavier Borg B.Eng.(Hons.) - Blaze Labs Research First electronic edition published as part of the Unified Theory Foundations (Feb 2005) [1] Abstract This paper shows that all measurable quantities in physics can be represented as nothing more than a number of spatial dimensions differentiated by a number of temporal dimensions and vice versa. To convert between the numerical values given by the space-time system of units and the conventional SI system, one simply multiplies the results by specific dimensionless constants. Once the ST system of units presented here is applied to any set of physics parameters, one is then able to derive all laws and equations without reference to the original theory which presented said relationship. In other words, all known principles and numerical constants which took hundreds of years to be discovered, like Ohm's Law, energy mass equivalence, Newton's Laws, etc.. would simply follow naturally from the spatial and temporal dimensions themselves, and can be derived without any reference to standard theoretical background. Hundreds of new equations can be derived using the ST table included in this paper. The relation between any combination of physical parameters, can be derived at any instant. Included is a step by step worked example showing how to derive any free space constant and quantum constant. 1 Dimensions and dimensional analysis One of the most powerful mathematical tools in science is dimensional analysis. Dimensional analysis is often applied in different scientific fields to simplify a problem by reducing the number of variables to the smallest number of "essential" parameters. -

Should Physical Laws Be Unit-Invariant?
Should physical laws be unit-invariant? 1. Introduction In a paper published in this journal in 2015, Sally Riordan reviews a recent debate about whether fundamental constants are really “constant”, or whether they may change over cosmological timescales. Her context is the impending redefinition of the kilogram and other units in terms of fundamental constants – a practice which is seen as more “objective” than defining units in terms of human artefacts. She focusses on one particular constant, the fine structure constant (α), for which some evidence of such a variation has been reported. Although α is not one of the fundamental constants involved in the proposed redefinitions, it is linked to some which are, so that there is a clear cause for concern. One of the authors she references is Michael Duff, who presents an argument supporting his assertion that dimensionless constants are more fundamental than dimensioned ones; this argument employs various systems of so-called “natural units”. [see Riordan (2015); Duff (2004)] An interesting feature of this discussion is that not only Riordan herself, but all the papers she cites, use a formula for α that is valid only in certain systems of units, and would not feature in a modern physics textbook. This violates another aspect of objectivity – namely, the idea that our physical laws should be expressed in such a way that they are independent of the particular units we choose to use; they should be unit-invariant. In this paper I investigate the place of unit-invariance in the history of physics, together with its converse, unit-dependence, which we will find is a common feature of some branches of physics, despite the fact that, as I will show in an analysis of Duff’s argument, unit-dependent formulae can lead to erroneous conclusions. -

The International System of Units (SI)
NAT'L INST. OF STAND & TECH NIST National Institute of Standards and Technology Technology Administration, U.S. Department of Commerce NIST Special Publication 330 2001 Edition The International System of Units (SI) 4. Barry N. Taylor, Editor r A o o L57 330 2oOI rhe National Institute of Standards and Technology was established in 1988 by Congress to "assist industry in the development of technology . needed to improve product quality, to modernize manufacturing processes, to ensure product reliability . and to facilitate rapid commercialization ... of products based on new scientific discoveries." NIST, originally founded as the National Bureau of Standards in 1901, works to strengthen U.S. industry's competitiveness; advance science and engineering; and improve public health, safety, and the environment. One of the agency's basic functions is to develop, maintain, and retain custody of the national standards of measurement, and provide the means and methods for comparing standards used in science, engineering, manufacturing, commerce, industry, and education with the standards adopted or recognized by the Federal Government. As an agency of the U.S. Commerce Department's Technology Administration, NIST conducts basic and applied research in the physical sciences and engineering, and develops measurement techniques, test methods, standards, and related services. The Institute does generic and precompetitive work on new and advanced technologies. NIST's research facilities are located at Gaithersburg, MD 20899, and at Boulder, CO 80303. -

AP Physics 2: Algebra Based
AP® PHYSICS 2 TABLE OF INFORMATION CONSTANTS AND CONVERSION FACTORS -27 -19 Proton mass, mp =1.67 ¥ 10 kg Electron charge magnitude, e =1.60 ¥ 10 C -27 -19 Neutron mass, mn =1.67 ¥ 10 kg 1 electron volt, 1 eV= 1.60 ¥ 10 J -31 8 Electron mass, me =9.11 ¥ 10 kg Speed of light, c =3.00 ¥ 10 m s Universal gravitational Avogadro’s number, N 6.02 1023 mol- 1 -11 3 2 0 = ¥ constant, G =6.67 ¥ 10 m kg s Acceleration due to gravity 2 Universal gas constant, R = 8.31 J (mol K) at Earth’s surface, g = 9.8 m s -23 Boltzmann’s constant, kB =1.38 ¥ 10 J K 1 unified atomic mass unit, 1 u=¥= 1.66 10-27 kg 931 MeV c2 -34 -15 Planck’s constant, h =¥=¥6.63 10 J s 4.14 10 eV s -25 3 hc =¥=¥1.99 10 J m 1.24 10 eV nm -12 2 2 Vacuum permittivity, e0 =8.85 ¥ 10 C N m 9 22 Coulomb’s law constant, k =1 4pe0 = 9.0 ¥ 10 N m C -7 Vacuum permeability, mp0 =4 ¥ 10 (T m) A -7 Magnetic constant, k¢ =mp0 4 = 1 ¥ 10 (T m) A 1 atmosphere pressure, 1 atm=¥= 1.0 1052 N m 1.0¥ 105 Pa meter, m mole, mol watt, W farad, F kilogram, kg hertz, Hz coulomb, C tesla, T UNIT second, s newton, N volt, V degree Celsius, ∞C SYMBOLS ampere, A pascal, Pa ohm, W electron volt, eV kelvin, K joule, J henry, H PREFIXES VALUES OF TRIGONOMETRIC FUNCTIONS FOR COMMON ANGLES Factor Prefix Symbol q 0 30 37 45 53 60 90 12 tera T 10 sinq 0 12 35 22 45 32 1 109 giga G cosq 1 32 45 22 35 12 0 106 mega M 103 kilo k tanq 0 33 34 1 43 3 • 10-2 centi c 10-3 milli m The following conventions are used in this exam. -

Approximate Molecular Electronic Structure Methods
APPROXIMATE MOLECULAR ELECTRONIC STRUCTURE METHODS By CHARLES ALBERT TAYLOR, JR. A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 1990 To my grandparents, Carrie B. and W. D. Owens ACKNOWLEDGMENTS It would be impossible to adequately acknowledge all of those who have contributed in one way or another to my “graduate school experience.” However, there are a few who have played such a pivotal role that I feel it appropriate to take this opportunity to thank them: • Dr. Michael Zemer for his enthusiasm despite my more than occasional lack thereof. • Dr. Yngve Ohm whose strong leadership and standards of excellence make QTP a truly unique environment for students, postdocs, and faculty alike. • Dr. Jack Sabin for coming to the rescue and providing me a workplace in which to finish this dissertation. I will miss your bookshelves which I hope are back in order before you read this. • Dr. William Weltner who provided encouragement and seemed to have confidence in me, even when I did not. • Joanne Bratcher for taking care of her boys and keeping things running smoothly at QTP-not to mention Sanibel. Of course, I cannot overlook those friends and colleagues with whom I shared much over the past years: • Alan Salter, the conservative conscience of the clubhouse and a good friend. • Bill Parkinson, the liberal conscience of the clubhouse who, along with his family, Bonnie, Ryan, Danny, and Casey; helped me keep things in perspective and could always be counted on for a good laugh. -

Physics and Technology System of Units for Electrodynamics
PHYSICS AND TECHNOLOGY SYSTEM OF UNITS FOR ELECTRODYNAMICS∗ M. G. Ivanov† Moscow Institute of Physics and Technology Dolgoprudny, Russia Abstract The contemporary practice is to favor the use of the SI units for electric circuits and the Gaussian CGS system for electromagnetic field. A modification of the Gaussian system of units (the Physics and Technology System) is suggested. In the Physics and Technology System the units of measurement for electrical circuits coincide with SI units, and the equations for the electromagnetic field are almost the same form as in the Gaussian system. The XXIV CGMP (2011) Resolution ¾On the possible future revision of the International System of Units, the SI¿ provides a chance to initiate gradual introduction of the Physics and Technology System as a new modification of the SI. Keywords: SI, International System of Units, electrodynamics, Physics and Technology System of units, special relativity. 1. Introduction. One and a half century dispute The problem of choice of units for electrodynamics dates back to the time of M. Faraday (1822–1831) and J. Maxwell (1861–1873). Electrodynamics acquired its final form only after geometrization of special relativity by H. Minkowski (1907–1909). The improvement of contemporary (4-dimentional relativistic covariant) formulation of electrodynamics and its implementation in practice of higher education stretched not less than a half of century. Overview of some systems of units, which are used in electrodynamics could be found, for example, in books [2, 3] and paper [4]. Legislation and standards of many countries recommend to use in science and education The International System of Units (SI). -
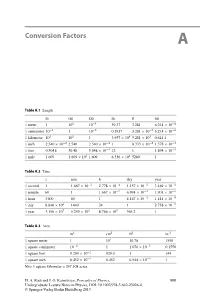
Conversion Factors A
Conversion Factors A Table A.1 Length mcmkmin.ftmi 1 meter 1 102 10−3 39.37 3.281 6.214 × 10−4 1 centimeter 10−2 110−5 0.3937 3.281 × 10−2 6.214 × 10−6 1 kilometer 103 105 13.937 × 104 3.281 × 103 0.621 4 1inch 2.540 × 10−2 2.540 2.540 × 10−5 18.333 × 10−2 1.578 × 10−5 1 foot 0.3048 30.48 3.048 × 10−4 12 1 1.894 × 10−4 1 mile 1 609 1.609 × 105 1.609 6.336 × 104 5280 1 Table A.2 Time s min h day year 1 second 1 1.667 × 10−2 2.778 × 10−4 1.157 × 10−5 3.169 × 10−8 1 minute 60 1 1.667 × 10−2 6.994 × 10−4 1.901 × 10−6 1 hour 3600 60 1 4.167 × 10−2 1.141 × 10−4 1day 8.640 × 104 1440 24 1 2.738 × 10−5 1 year 3.156 × 107 5.259 × 105 8.766 × 103 365.2 1 Table A.3 Area m2 cm2 ft2 in.2 1 square meter 1 104 10.76 1550 1 square centimeter 10−4 11.076 × 10−3 0.1550 1 square foot 9.290 × 10−2 929.0 1 144 1 square inch 6.452 × 10−4 6.452 6.944 × 10−3 1 Note 1 square kilometer = 247.108 acres H. A. Radi and J. O. Rasmussen, Principles of Physics, 999 Undergraduate Lecture Notes in Physics, DOI: 10.1007/978-3-642-23026-4, © Springer-Verlag Berlin Heidelberg 2013 1000 A Conversion Factors Table A.4 Volume m3 cm3 Lft3 in.3 1 cubic meter 1 106 1000 35.51 6.102 × 104 1 cubic centimeter 10−6 11.000 × 10−3 3.531 × 10−5 6.102 × 10−2 1liter 1.000 × 10−3 1000 1 3.531 × 10−2 61.02 1 cubic foot 2.832 × 10−4 1 28.32 1 1728 1 cubic inch 1.639 × 10−4 16.39 1.639 × 10−2 5.787 × 10−4 1 Note 1 U.S. -

The International System of Units (SI) - Conversion Factors For
NIST Special Publication 1038 The International System of Units (SI) – Conversion Factors for General Use Kenneth Butcher Linda Crown Elizabeth J. Gentry Weights and Measures Division Technology Services NIST Special Publication 1038 The International System of Units (SI) - Conversion Factors for General Use Editors: Kenneth S. Butcher Linda D. Crown Elizabeth J. Gentry Weights and Measures Division Carol Hockert, Chief Weights and Measures Division Technology Services National Institute of Standards and Technology May 2006 U.S. Department of Commerce Carlo M. Gutierrez, Secretary Technology Administration Robert Cresanti, Under Secretary of Commerce for Technology National Institute of Standards and Technology William Jeffrey, Director Certain commercial entities, equipment, or materials may be identified in this document in order to describe an experimental procedure or concept adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the entities, materials, or equipment are necessarily the best available for the purpose. National Institute of Standards and Technology Special Publications 1038 Natl. Inst. Stand. Technol. Spec. Pub. 1038, 24 pages (May 2006) Available through NIST Weights and Measures Division STOP 2600 Gaithersburg, MD 20899-2600 Phone: (301) 975-4004 — Fax: (301) 926-0647 Internet: www.nist.gov/owm or www.nist.gov/metric TABLE OF CONTENTS FOREWORD.................................................................................................................................................................v -
Physics Formulas List
Physics Formulas List Home Physics 95 Physics Formulas 23 Listen Physics Formulas List Learning physics is all about applying concepts to solve problems. This article provides a comprehensive physics formulas list, that will act as a ready reference, when you are solving physics problems. You can even use this list, for a quick revision before an exam. Physics is the most fundamental of all sciences. It is also one of the toughest sciences to master. Learning physics is basically studying the fundamental laws that govern our universe. I would say that there is a lot more to ascertain than just remember and mug up the physics formulas. Try to understand what a formula says and means, and what physical relation it expounds. If you understand the physical concepts underlying those formulas, deriving them or remembering them is easy. This Buzzle article lists some physics formulas that you would need in solving basic physics problems. Physics Formulas Mechanics Friction Moment of Inertia Newtonian Gravity Projectile Motion Simple Pendulum Electricity Thermodynamics Electromagnetism Optics Quantum Physics Derive all these formulas once, before you start using them. Study physics and look at it as an opportunity to appreciate the underlying beauty of nature, expressed through natural laws. Physics help is provided here in the form of ready to use formulas. Physics has a reputation for being difficult and to some extent that's http://www.buzzle.com/articles/physics-formulas-list.html[1/10/2014 10:58:12 AM] Physics Formulas List true, due to the mathematics involved. If you don't wish to think on your own and apply basic physics principles, solving physics problems is always going to be tough. -

Unit Systems, E&M Units, and Natural Units
Phys 239 Quantitative Physics Lecture 4: Units Unit Systems, E&M Units, and Natural Units Unit Soup We live in a soup of units—especially in the U.S. where we are saddled with imperial units that even the former empire has now dropped. I was once—like many scientists and most international students in the U.S. are today—vocally irritated and perplexed by this fact. Why would the U.S.—who likes to claim #1 status in all things even when not true—be behind the global curve here, when others have transitioned? I only started to understand when I learned machining practices in grad school. The U.S. has an immense infrastructure for manufacturing, comprised largely of durable machines that last a lifetime. The machines and tools represent a substantial capital investment not easily brushed aside. It is therefore comparatively harder for a (former?) manufacturing powerhouse to make the change. So we become adept in two systems. As new machines often build in dual capability, we may yet see a transformation decades away. But even leaving aside imperial units, scientists bicker over which is the “best” metric-flavored unit system. This tends to break up a bit by generation and by field. Younger scientists tend to have been trained in SI units, also known as MKSA for Meters, Kilograms, Seconds, and Amperes. Force, energy, and power become Newtons, Joules, and Watts. Longer-toothed scientists often prefer c.g.s. (centimeter, gram, seconds) in conjunction with Gaussian units for electromagnetic problems. Force, energy, and power become dynes, ergs, and ergs/second.