Unit Systems, E&M Units, and Natural Units
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
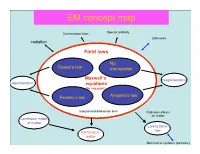
EM Concept Map
EM concept map Conservation laws Special relativity EM waves radiation Field laws No Gauss’s law monopoles ConservationMaxwell’s laws magnetostatics electrostatics equations (In vacuum) Faraday’s law Ampere’s law Integral and differential form Radiation effects on matter Continuum model of matter Lorenz force law EM fields in matter Mechanical systems (particles) Electricity and Magnetism PHYS-350: (updated Sept. 9th, (1605-1725 Monday, Wednesday, Leacock 109) 2010) Instructor: Shaun Lovejoy, Rutherford Physics, rm. 213, local Outline: 6537, email: [email protected]. 1. Vector Analysis: Tutorials: Tuesdays 4:15pm-5:15pm, location: the “Boardroom” Algebra, differential and integral calculus, curvilinear coordinates, (the southwest corner, ground floor of Rutherford physics). Office Hours: Thursday 4-5pm (either Lhermitte or Gervais, see Dirac function, potentials. the schedule on the course site). 2. Electrostatics: Teaching assistant: Julien Lhermitte, rm. 422, local 7033, email: Definitions, basic notions, laws, divergence and curl of the electric [email protected] potential, work and energy. Gervais, Hua Long, ERP-230, [email protected] 3. Special techniques: Math background: Prerequisites: Math 222A,B (Calculus III= Laplace's equation, images, seperation of variables, multipole multivariate calculus), 223A,B (Linear algebra), expansion. Corequisites: 314A (Advanced Calculus = vector 4. Electrostatic fields in matter: calculus), 315A (Ordinary differential equations) Polarization, electric displacement, dielectrics. Primary Course Book: "Introduction to Electrodynamics" by D. 5. Magnetostatics: J. Griffiths, Prentice-Hall, (1999, third edition). Lorenz force law, Biot-Savart law, divergence and curl of B, vector Similar books: potentials. -“Electromagnetism”, G. L. Pollack, D. R. Stump, Addison and 6. Magnetostatic fields in matter: Wesley, 2002. -

New Realization of the Ohm and Farad Using the NBS Calculable Capacitor
IEEE TRANSACTIONS ON INSTRUMENTATION AND MEASUREMENT, VOL. 38, NO. 2, APRIL 1989 249 New Realization of the Ohm and Farad Using the NBS Calculable Capacitor JOHN Q. SHIELDS, RONALD F. DZIUBA, MEMBER, IEEE,AND HOWARD P. LAYER Abstrucl-Results of a new realization of the ohm and farad using part of the NBS effort. This paper describes our measure- the NBS calculable capacitor and associated apparatus are reported. ments and gives our latest results. The results show that both the NBS representation of the ohm and the NBS representation of the farad are changing with time, cNBSat the rate of -0.054 ppmlyear and FNssat the rate of 0.010 ppm/year. The 11. AC MEASUREMENTS realization of the ohm is of particular significance at this time because The measurement sequence used in the 1974 ohm and of its role in assigning an SI value to the quantized Hall resistance. The farad determinations [2] has been retained in the present estimated uncertainty of the ohm realization is 0.022 ppm (lo) while the estimated uncertainty of the farad realization is 0.014 ppm (la). NBS measurements. A 0.5-pF calculable cross-capacitor is used to measure a transportable 10-pF reference capac- itor which is carried to the laboratory containing the NBS I. INTRODUCTION bank of 10-pF fused silica reference capacitors. A 10: 1 HE NBS REPRESENTATION of the ohm is bridge is used in two stages to measure two 1000-pF ca- T based on the mean resistance of five Thomas-type pacitors which are in turn used as two arms of a special wire-wound resistors maintained in a 25°C oil bath at NBS frequency-dependent bridge for measuring two 100-kQ Gaithersburg, MD. -

An Atomic Physics Perspective on the New Kilogram Defined by Planck's Constant
An atomic physics perspective on the new kilogram defined by Planck’s constant (Wolfgang Ketterle and Alan O. Jamison, MIT) (Manuscript submitted to Physics Today) On May 20, the kilogram will no longer be defined by the artefact in Paris, but through the definition1 of Planck’s constant h=6.626 070 15*10-34 kg m2/s. This is the result of advances in metrology: The best two measurements of h, the Watt balance and the silicon spheres, have now reached an accuracy similar to the mass drift of the ur-kilogram in Paris over 130 years. At this point, the General Conference on Weights and Measures decided to use the precisely measured numerical value of h as the definition of h, which then defines the unit of the kilogram. But how can we now explain in simple terms what exactly one kilogram is? How do fixed numerical values of h, the speed of light c and the Cs hyperfine frequency νCs define the kilogram? In this article we give a simple conceptual picture of the new kilogram and relate it to the practical realizations of the kilogram. A similar change occurred in 1983 for the definition of the meter when the speed of light was defined to be 299 792 458 m/s. Since the second was the time required for 9 192 631 770 oscillations of hyperfine radiation from a cesium atom, defining the speed of light defined the meter as the distance travelled by light in 1/9192631770 of a second, or equivalently, as 9192631770/299792458 times the wavelength of the cesium hyperfine radiation. -

Operating Instructions Ac Snap-Around Volt-Ohm
4.5) HOW TO USE POINTER LOCK & RANGE FINDER LIFETIME LIMITED WARRANTY 05/06 From #174-1 SYMBOLS The attention to detail of this fine snap-around instrument is further enhanced by OPERATING INSTRUCTIONS (1) Slide the pointer lock button to the left. This allows easy readings in dimly lit the application of Sperry's unmatched service and concern for detail and or crowded cable areas (Fig.8). reliability. AC SNAP-AROUND VOLT-OHM-AMMETER (2) For quick and easy identification the dial drum is marked with the symbols as These Sperry snap-arounds are internationally accepted by craftsman and illustrated below (Fig.9). servicemen for their unmatched performance. All Sperry's snap-around MODEL SPR-300 PLUS & SPR-300 PLUS A instruments are unconditionally warranted against defects in material and Pointer Ampere Higher workmanship under normal conditions of use and service; our obligations under Lock range range to the to the this warranty being limited to repairing or replacing, free of charge, at Sperry's right. right. sole option, any such Sperry snap-around instrument that malfunctions under normal operating conditions at rated use. 1 Lower Lower range range to the to the left. left. REPLACEMENT PROCEDURE Fig.8 Fig.9 Securely wrap the instrument and its accessories in a box or mailing bag and ship prepaid to the address below. Be sure to include your name and address, as 5) BATTERY & FUSE REPLACEMENT well as the name of the distributor, with a copy of your invoice from whom the (1) Remove the screw on the back of the case for battery and fuse replacement unit was purchased, clearly identifying the model number and date of purchase. -

Modern Physics Unit 15: Nuclear Structure and Decay Lecture 15.1: Nuclear Characteristics
Modern Physics Unit 15: Nuclear Structure and Decay Lecture 15.1: Nuclear Characteristics Ron Reifenberger Professor of Physics Purdue University 1 Nucleons - the basic building blocks of the nucleus Also written as: 7 3 Li ~10-15 m Examples: A X=Chemical Element Z X Z = number of protons = atomic number 12 A = atomic mass number = Z+N 6 C N= A-Z = number of neutrons 35 17 Cl 2 What is the size of a nucleus? Three possibilities • Range of nuclear force? • Mass radius? • Charge radius? It turns out that for nuclear matter Nuclear force radius ≈ mass radius ≈ charge radius defines nuclear force range defines nuclear surface 3 Nuclear Charge Density The size of the lighter nuclei can be approximated by modeling the nuclear charge density ρ (C/m3): t ≈ 4.4a; a=0.54 fm 90% 10% Usually infer the best values for ρo, R and a for a given nucleus from scattering experiments 4 Nuclear mass density Scattering experiments indicate the nucleus is roughly spherical with a radius given by 1 3 −15 R = RRooA ; =1.07 × 10meters= 1.07 fm = 1.07 fermis A=atomic mass number What is the nuclear mass density of the most common isotope of iron? 56 26 Fe⇒= A56; Z = 26, N = 30 m Am⋅⋅3 Am 33A ⋅ m m ρ = nuc p= p= pp = o 1 33 VR4 3 3 3 44ππA R nuc π R 4(π RAo ) o o 3 3×× 1.66 10−27 kg = 3.2× 1017kg / m 3 4×× 3.14 (1.07 × 10−15m ) 3 The mass density is constant, independent of A! 5 Nuclear mass density for 27Al, 97Mo, 238U (from scattering experiments) (kg/m3) ρo heavy mass nucleus light mass nucleus middle mass nucleus 6 Typical Densities Material Density Helium 0.18 kg/m3 Air (dry) 1.2 kg/m3 Styrofoam ~100 kg/m3 Water 1000 kg/m3 Iron 7870 kg/m3 Lead 11,340 kg/m3 17 3 Nuclear Matter ~10 kg/m 7 Isotopes - same chemical element but different mass (J.J. -

Gauss' Linking Number Revisited
October 18, 2011 9:17 WSPC/S0218-2165 134-JKTR S0218216511009261 Journal of Knot Theory and Its Ramifications Vol. 20, No. 10 (2011) 1325–1343 c World Scientific Publishing Company DOI: 10.1142/S0218216511009261 GAUSS’ LINKING NUMBER REVISITED RENZO L. RICCA∗ Department of Mathematics and Applications, University of Milano-Bicocca, Via Cozzi 53, 20125 Milano, Italy [email protected] BERNARDO NIPOTI Department of Mathematics “F. Casorati”, University of Pavia, Via Ferrata 1, 27100 Pavia, Italy Accepted 6 August 2010 ABSTRACT In this paper we provide a mathematical reconstruction of what might have been Gauss’ own derivation of the linking number of 1833, providing also an alternative, explicit proof of its modern interpretation in terms of degree, signed crossings and inter- section number. The reconstruction presented here is entirely based on an accurate study of Gauss’ own work on terrestrial magnetism. A brief discussion of a possibly indepen- dent derivation made by Maxwell in 1867 completes this reconstruction. Since the linking number interpretations in terms of degree, signed crossings and intersection index play such an important role in modern mathematical physics, we offer a direct proof of their equivalence. Explicit examples of its interpretation in terms of oriented area are also provided. Keywords: Linking number; potential; degree; signed crossings; intersection number; oriented area. Mathematics Subject Classification 2010: 57M25, 57M27, 78A25 1. Introduction The concept of linking number was introduced by Gauss in a brief note on his diary in 1833 (see Sec. 2 below), but no proof was given, neither of its derivation, nor of its topological meaning. Its derivation remained indeed a mystery. -

Musings on the Planck Length Universe
Musings on the Planck Length Universe -How does matter, or how does a change move through space on the micro- level? The answer depends on how the structure of space and matter are defined at a certain order of magnitude and what are their properties at that level. We will assume that, on the micro-level, the limits of space are discrete, consisting of the smallest units possible. Therefore, in order to define the structure of space on this level it would be necessary to introduce a notion of an “elementary spatial unit” (ESU), the smallest part of space, some kind of spatial “atom”. This would be the smallest and indivisible finite part (cell) of space, defined only by size and position (neighborhoods). It doesn’t have any content, but it could be filled with some property like “matter” or “vacuum”. There are two possibilities in which a property can to move through space (change its position). One would be that an ESU itself, containing a certain property, moves between other ESU carrying its content like a fish moving through the water or a ball through the air. Another possibility would be that the positions of all the ESU don’t change while their content is moving. This would be like an old billboard covered with light bulbs, or a screen with pixels. The positions of the bulbs or pixels are fixed, and only their content (light) changes position from one bulb to the other. Here the spatial structure (the structure of positions) is fixed and it is only the property (the content) that changes positions, thus moving through space. -

Dimensional Analysis and the Theory of Natural Units
LIBRARY TECHNICAL REPORT SECTION SCHOOL NAVAL POSTGRADUATE MONTEREY, CALIFORNIA 93940 NPS-57Gn71101A NAVAL POSTGRADUATE SCHOOL Monterey, California DIMENSIONAL ANALYSIS AND THE THEORY OF NATURAL UNITS "by T. H. Gawain, D.Sc. October 1971 lllp FEDDOCS public This document has been approved for D 208.14/2:NPS-57GN71101A unlimited release and sale; i^ distribution is NAVAL POSTGRADUATE SCHOOL Monterey, California Rear Admiral A. S. Goodfellow, Jr., USN M. U. Clauser Superintendent Provost ABSTRACT: This monograph has been prepared as a text on dimensional analysis for students of Aeronautics at this School. It develops the subject from a viewpoint which is inadequately treated in most standard texts hut which the author's experience has shown to be valuable to students and professionals alike. The analysis treats two types of consistent units, namely, fixed units and natural units. Fixed units include those encountered in the various familiar English and metric systems. Natural units are not fixed in magnitude once and for all but depend on certain physical reference parameters which change with the problem under consideration. Detailed rules are given for the orderly choice of such dimensional reference parameters and for their use in various applications. It is shown that when transformed into natural units, all physical quantities are reduced to dimensionless form. The dimension- less parameters of the well known Pi Theorem are shown to be in this category. An important corollary is proved, namely that any valid physical equation remains valid if all dimensional quantities in the equation be replaced by their dimensionless counterparts in any consistent system of natural units. -

Units and Magnitudes (Lecture Notes)
physics 8.701 topic 2 Frank Wilczek Units and Magnitudes (lecture notes) This lecture has two parts. The first part is mainly a practical guide to the measurement units that dominate the particle physics literature, and culture. The second part is a quasi-philosophical discussion of deep issues around unit systems, including a comparison of atomic, particle ("strong") and Planck units. For a more extended, profound treatment of the second part issues, see arxiv.org/pdf/0708.4361v1.pdf . Because special relativity and quantum mechanics permeate modern particle physics, it is useful to employ units so that c = ħ = 1. In other words, we report velocities as multiples the speed of light c, and actions (or equivalently angular momenta) as multiples of the rationalized Planck's constant ħ, which is the original Planck constant h divided by 2π. 27 August 2013 physics 8.701 topic 2 Frank Wilczek In classical physics one usually keeps separate units for mass, length and time. I invite you to think about why! (I'll give you my take on it later.) To bring out the "dimensional" features of particle physics units without excess baggage, it is helpful to keep track of powers of mass M, length L, and time T without regard to magnitudes, in the form When these are both set equal to 1, the M, L, T system collapses to just one independent dimension. So we can - and usually do - consider everything as having the units of some power of mass. Thus for energy we have while for momentum 27 August 2013 physics 8.701 topic 2 Frank Wilczek and for length so that energy and momentum have the units of mass, while length has the units of inverse mass. -

Summary of Changes for Bidder Reference. It Does Not Go Into the Project Manual. It Is Simply a Reference of the Changes Made in the Frontal Documents
Community Colleges of Spokane ALSC Architects, P.S. SCC Lair Remodel 203 North Washington, Suite 400 2019-167 G(2-1) Spokane, WA 99201 ALSC Job No. 2019-010 January 10, 2020 Page 1 ADDENDUM NO. 1 The additions, omissions, clarifications and corrections contained herein shall be made to drawings and specifications for the project and shall be included in scope of work and proposals to be submitted. References made below to specifications and drawings shall be used as a general guide only. Bidder shall determine the work affected by Addendum items. General and Bidding Requirements: 1. Pre-Bid Meeting Pre-Bid Meeting notes and sign in sheet attached 2. Summary of Changes Summary of Changes for bidder reference. It does not go into the Project Manual. It is simply a reference of the changes made in the frontal documents. It is not a part of the construction documents. In the Specifications: 1. Section 00 30 00 REPLACED section in its entirety. See Summary of Instruction to Bidders Changes for description of changes 2. Section 00 60 00 REPLACED section in its entirety. See Summary of General and Supplementary Conditions Changes for description of changes 3. Section 00 73 10 DELETE section in its entirety. Liquidated Damages Checklist Mead School District ALSC Architects, P.S. New Elementary School 203 North Washington, Suite 400 ALSC Job No. 2018-022 Spokane, WA 99201 March 26, 2019 Page 2 ADDENDUM NO. 1 4. Section 23 09 00 Section 2.3.F CHANGED paragraph to read “All Instrumentation and Control Systems controllers shall have a communication port for connections with the operator interfaces using the LonWorks Data Link/Physical layer protocol.” A. -

Six Easy Roads to the Planck Scale
Six easy roads to the Planck scale Ronald J. Adler∗ Hansen Laboratory for Experimental Physics Gravity Probe B Mission, Stanford University, Stanford California 94309 Abstract We give six arguments that the Planck scale should be viewed as a fundamental minimum or boundary for the classical concept of spacetime, beyond which quantum effects cannot be neglected and the basic nature of spacetime must be reconsidered. The arguments are elementary, heuristic, and plausible, and as much as possible rely on only general principles of quantum theory and gravity theory. The paper is primarily pedagogical, and its main goal is to give physics students, non-specialists, engineers etc. an awareness and appreciation of the Planck scale and the role it should play in present and future theories of quantum spacetime and quantum gravity. arXiv:1001.1205v1 [gr-qc] 8 Jan 2010 1 I. INTRODUCTION Max Planck first noted in 18991,2 the existence of a system of units based on the three fundamental constants, G = 6:67 × 10−11Nm2=kg2(or m3=kg s2) (1) c = 3:00 × 108m=s h = 6:60 × 10−34Js(or kg m2=s) These constants are dimensionally independent in the sense that no combination is dimen- sionless and a length, a time, and a mass, may be constructed from them. Specifically, using ~ ≡ h=2π = 1:05 × 10−34Js in preference to h, the Planck scale is r r G G l = ~ = 1:6 × 10−35m; T = ~ = 0:54 × 10−43; (2) P c3 P c5 r c M = ~ = 2:2 × 10−8kg P G 2 19 3 The energy associated with the Planck mass is EP = MP c = 1:2 × 10 GeV . -

Probing the Minimal Length Scale by Precision Tests of the Muon G − 2
Physics Letters B 584 (2004) 109–113 www.elsevier.com/locate/physletb Probing the minimal length scale by precision tests of the muon g − 2 U. Harbach, S. Hossenfelder, M. Bleicher, H. Stöcker Institut für Theoretische Physik, J.W. Goethe-Universität, Robert-Mayer-Str. 8-10, 60054 Frankfurt am Main, Germany Received 25 November 2003; received in revised form 20 January 2004; accepted 21 January 2004 Editor: P.V. Landshoff Abstract Modifications of the gyromagnetic moment of electrons and muons due to a minimal length scale combined with a modified fundamental scale Mf are explored. First-order deviations from the theoretical SM value for g − 2 due to these string theory- motivated effects are derived. Constraints for the fundamental scale Mf are given. 2004 Elsevier B.V. All rights reserved. String theory suggests the existence of a minimum • the need for a higher-dimensional space–time and length scale. An exciting quantum mechanical impli- • the existence of a minimal length scale. cation of this feature is a modification of the uncer- tainty principle. In perturbative string theory [1,2], Naturally, this minimum length uncertainty is re- the feature of a fundamental minimal length scale lated to a modification of the standard commutation arises from the fact that strings cannot probe dis- relations between position and momentum [6,7]. Ap- tances smaller than the string scale. If the energy of plication of this is of high interest for quantum fluc- a string reaches the Planck mass mp, excitations of the tuations in the early universe and inflation [8–16]. string can occur and cause a non-zero extension [3].