The Fossil Loon, Colymboides Minutes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Point Reyes National Seashore Bird List
Birds of Point Reyes National Seashore Gaviidae (Loons) Alcedinidae (Kingfishers) Podicipedidae (Grebes) Picidae (Woodpeckers) Diomedeidae (Albatrosses) Tyrannidae (Tyrant Flycatcher) Procellariidae (Shearwaters, Petrels) Alaudidae (Larks) Hydrobatidae (Storm Petrels) Hirundinidae (Swallows) Sulidae (Boobies, Gannets) Laniidae (Shrikes) Pelecanidae (Pelicans) Vireonidae (Vireos) Phalacrocoracidae (Cormorants) Corvidae (Crows, Jays) Fregatidae (Frigate Birds) Paridae (Chickadees, Titmice) Ardeidae (Herons, Bitterns, & Egrets) Aegithalidae (Bushtits) Threskiornithidae (Ibises, Spoonbills) Sittidae (Nuthatches) Ciconiidae (Storks) Certhiidae (Creepers) Anatidae (Ducks, Geese, Swans) Troglodytidae (Wrens) Cathartidae (New World Vultures) Cinclidae (Dippers) Accipitridae (Hawks, Kites, Eagles) & Regulidae (Kinglets) Falconidae (Caracaras, Falcons) Sylviidae (Old World Warblers, Gnatcatchers) Odontophoridae (New World Quail) Turdidae (Thrushes) Rallidae (Rails, Gallinules, Coots) Timaliidae (Babblers) Gruidae (Cranes) Mimidae (Mockingbirds, Thrashers) Charadriidae (Lapwings, Plovers) Motacillidae (Wagtails, Pipits) Haematopodidae (Oystercatcher) Bombycillidae (Waxwings) Recurvirostridae (Stilts, Avocets) Ptilogonatidae (Silky-flycatcher) Scolopacidae (Sandpipers, Phalaropes) Parulidae (Wood Warblers) Laridae (Skuas, Gulls, Terns, Skimmers) Cardinalidae (Cardinals) Alcidae (Auks, Murres, Puffins) Emberizidae (Emberizids) Columbidae (Pigeons, Doves) Fringillidae (Finches) Cuculidae (Cuckoos, Road Runners, Anis) NON-NATIVES Tytonidae (Barn Owls) -

The Cycle of the Common Loon (Brochure)
ADIRONDACK LOONS AND LAKES FOR MORE INFORMATION: NEED YOUR HELP! lthough the Adirondack Park provides A suitable habitat for breeding loons, the summering population in the Park still faces many challenges. YOU CAN HELP! WCS’ Adirondack Loon Conservation Program Keep Shorelines Natural: Help maintain ~The Cycle of the this critical habitat for nesting wildlife and 7 Brandy Brook Ave, Suite 204 for the quality of our lake water. Saranac Lake, NY 12983 Common Loon~ (518) 891-8872, [email protected] Out on a Lake? Keep your distance (~100 feet or more) from loons and other wildlife, www.wcs.org/adirondackloons so that you do not disturb them. The Wildlife Conservation Society’s Adirondack Going Fishing? Loon Conservation Program is dedicated to ∗ Use Non-Lead Fishing Sinkers and improving the overall health of the environment, Jigs. Lead fishing tackle is poisonous to particularly the protection of air and water loons and other wildlife when quality, through collaborative research and accidentally ingested. education efforts focusing on the natural history ∗ Pack Out Your Line. Invisible in the of the Common Loon (Gavia immer) and water, lost or cut fishing line can conservation issues affecting loon populations entangle loons and other wildlife, often and their aquatic habitats. with fatal results. THE WILDLIFE CONSERVATION SOCIETY IS Be an Environmentally Wise Consumer: GRATEFUL TO ITS COLLABORATORS FOR THEIR Many forms of environmental pollution SUPPORT OF THE LOON PROGRAM: result from the incineration of fossil Natural History Museum of the Adirondacks - fuels, primarily from coal-fired power The W!ld Center plants and vehicles, negatively affecting www.wildcenter.org A guide to the seasonal Adirondack ecosystems and their wild NYS Dept. -
![LOONS and GREBES [ ] Common Loon [ ] Pied-Billed Grebe---X](https://docslib.b-cdn.net/cover/1406/loons-and-grebes-common-loon-pied-billed-grebe-x-351406.webp)
LOONS and GREBES [ ] Common Loon [ ] Pied-Billed Grebe---X
LOONS and GREBES [ ] Common Merganser [ ] Common Loon [ ] Ruddy Duck---x OWLS [ ] Pied-billed Grebe---x [ ] Barn Owl [ ] Horned Grebe HAWKS, KITES and EAGLES [ ] Eared Grebe [ ] Northern Harrier SWIFTS and HUMMINGBIRDS [ ] Western Grebe [ ] Cooper’s Hawk [ ] White-throated Swift [ ] Clark’s Grebe [ ] Red-shouldered Hawk [ ] Anna’s Hummingbird---x [ ] Red-tailed Hawk KINGFISHERS PELICANS and CORMORANTS [ ] Golden Eagle [ ] Belted Kingfisher [ ] Brown Pelican [ ] American Kestrel [ ] Double-crested Cormorant [ ] White-tailed Kite WOODPECKERS [ ] Acorn Woodpecker BITTERNS, HERONS and EGRETS PHEASANTS and QUAIL [ ] Red-breasted Sapsucker [ ] American Bittern [ ] Ring-necked Pheasant [ ] Nuttall’s Woodpecker---x [ ] Great Blue Heron [ ] California Quail [ ] Great Egret [ ] Downy Woodpecker [ ] Snowy Egret RAILS [ ] Northern Flicker [ ] Sora [ ] Green Heron---x TYRANT FLYCATCHERS [ ] Black-crowned Night-Heron---x [ ] Common Moorhen Pacific-slope Flycatcher [ ] American Coot---x [ ] NEW WORLD VULTURES [ ] Black Phoebe---x Say’s Phoebe [ ] Turkey Vulture SHOREBIRDS [ ] [ ] Killdeer---x [ ] Ash-throated Flycatcher WATERFOWL [ ] Greater Yellowlegs [ ] Western Kingbird [ ] Greater White-fronted Goose [ ] Black-necked Stilt SHRIKES [ ] Ross’s Goose [ ] Spotted Sandpiper Loggerhead Shrike [ ] Canada Goose---x [ ] Least Sandpiper [ ] [ ] Wood Duck [ ] Long-billed Dowitcher VIREOS Gadwall [ ] [ ] Wilson’s Snipe [ ] Warbling Vireo [ ] American Wigeon [ ] Mallard---x GULLS and TERNS JAYS and CROWS [ ] Cinnamon Teal [ ] Mew Gull [ ] Western Scrub-Jay---x -

Common Birds of the Estero Bay Area
Common Birds of the Estero Bay Area Jeremy Beaulieu Lisa Andreano Michael Walgren Introduction The following is a guide to the common birds of the Estero Bay Area. Brief descriptions are provided as well as active months and status listings. Photos are primarily courtesy of Greg Smith. Species are arranged by family according to the Sibley Guide to Birds (2000). Gaviidae Red-throated Loon Gavia stellata Occurrence: Common Active Months: November-April Federal Status: None State/Audubon Status: None Description: A small loon seldom seen far from salt water. In the non-breeding season they have a grey face and red throat. They have a long slender dark bill and white speckling on their dark back. Information: These birds are winter residents to the Central Coast. Wintering Red- throated Loons can gather in large numbers in Morro Bay if food is abundant. They are common on salt water of all depths but frequently forage in shallow bays and estuaries rather than far out at sea. Because their legs are located so far back, loons have difficulty walking on land and are rarely found far from water. Most loons must paddle furiously across the surface of the water before becoming airborne, but these small loons can practically spring directly into the air from land, a useful ability on its artic tundra breeding grounds. Pacific Loon Gavia pacifica Occurrence: Common Active Months: November-April Federal Status: None State/Audubon Status: None Description: The Pacific Loon has a shorter neck than the Red-throated Loon. The bill is very straight and the head is very smoothly rounded. -

Final Restoration Plan for Common Loon and Other Birds Impacted by the Bouchard Barge 120 (B-120) Oil Spill, Buzzards Bay Massachusetts and Rhode Island
FINAL RESTORATION PLAN for COMMON LOON (Gavia immer) and OTHER BIRDS IMPACTED BY THE BOUCHARD BARGE 120 (B-120) OIL SPILL BUZZARDS BAY MASSACHUSETTS and RHODE ISLAND June 2020 Prepared by: United States Fish and Wildlife Service Massachusetts Executive Office of Energy and Environmental Affairs Rhode Island Department of Environmental Management and National Oceanic and Atmospheric Administration (Lead Administrative Trustee) Executive Summary In April 2003, the Bouchard Barge‐120 (B‐120) oil spill (the Spill) affected more than 100 miles of Buzzards Bay and its shoreline and nearby coastal waters in both Massachusetts (MA) and Rhode Island (RI). Birds were exposed to and ingested oil as they foraged, nested, and/or migrated through the area. Species of birds estimated to have been killed in the greatest numbers included common loon (Gavia immer), common and roseate terns (Sterna hirundo and Sterna dougallii), and other birds such as common eider (Somateria mollissima), black scoter (Melanitta americana), and red‐throated loon (Gavia stellata). The National Oceanic and Atmospheric Administration (NOAA), U.S. Department of the Interior (DOI) (acting through the U.S. Fish and Wildlife Service [USFWS]), the Commonwealth of Massachusetts (acting through the Executive Office of Energy and Environmental Affairs [EEA]), and the State of Rhode Island serve as the natural resource Trustees (Trustees) responsible under the Oil Pollution Act of 1990 (OPA) (33 U.S.C. § 2701, et seq.) for ensuring the natural resources injured from the Spill are restored. As a designated Trustee, each agency is authorized to act on behalf of the public under State1 and/or Federal law to assess and recover natural resource damages, and to plan and implement actions to restore, rehabilitate, replace, or acquire the equivalent of the natural resources or services injured or lost as a result of an unpermitted discharge of oil. -

The Common Loon in New York State
THE COMMON LOON IN NEW YORK STATE For a number of years a committee of the Federation of New York State Bird Clubs has been planning a new Birds of New York State to succeed the monumental two volume work by E. H. Eaton, published by the State Museum a half century ago. This new book is not envisioned as an all-encompassing state book in the classic tradition. The day is long past for lavish volumes, liberally illus- trated, describing and portraying in detail the plumages, the habits, the life histories, and the economic importance of each species ever recorded in the state, just as if there were no other readily-available sources for this informa- tion. The committee believes that what a present-day state book should do is to concentrate on what is specifically germane to the ornithology of the state. It should be an inventory of the state list, a census of the breeding species, an estimate of populations wherever possible, a guide to the seasonal calendar of the species found in New York, an atlas of breeding ranges and migration routes, and an accounting of the unusual species that have been recorded within our boundaries. It should additionally give an accounting of the species normally to be found in the various ecological associations of the state. All this to give as accurate as possible a picture, and historical record, of the bird life of the state as it exists just prior to publication. It should certainly record changes that have taken place over the years, when known, and might even venture a prediction or two about the future. -

Beached Bird Guide for Northern Lake Michigan
Beached Bird Guide for Northern Lake Michigan Prepared by Common Coast Research & Conservation In association with the Grand Traverse Bay Botulism Network © 2008 Common Coast Research & Conservation How to use this guide This guide was developed to aid with the field identification of the most common waterbird species implicated in botulism E die-offs on northern Lake Michigan. The guide is not intended to be a comprehensive treatment of all species you may encounter in the field. For birds not treated in this guide please document with photographs and/or submit carcasses to the nearest Michigan Department of Natural Resources Field Office for identification and/or testing for botulism (see manual). The emphasis of this guide is on differences in bill structure among the various waterbird species. The bill plates are drawn to actual size - we recommend laminating the guide for use in the field. Placing the bills of unknown species directly on the plates will facilitate identification. Please keep in mind some variation among individuals is to be expected. Photographs of unknown species are helpful for later identification. Bird Topography tarsus crown bill (upper and lower mandibles) foot bill margin cheek throat wing coverts (lesser) secondaries webbed foot lobed foot primaries (loons, ducks, gulls) (grebes) Loons and Grebes Birds with dagger-like bills Description: Adult Common Loon bill large, dagger-like, mandible edges smooth feet webbed tarsus narrow, flat Plumage variation (adult vs. juvenile): Look at wing coverts: Adult – well-defined white "windows" (see photo) Juvenile - lacks defined white "windows" Similar species: Red-throated Loon – bill smaller (rarely found) Red-necked Grebe – feet lobed, bill smaller Description: Red-throated Loon bill dagger-like, slightly upturned, mandible edges smooth feet webbed tarsus narrow, flat Similar species: Common Loon - larger; bill heavier, not upturned Red-necked Grebe – feet lobed , bill yellowish NOTE: Rarely encountered. -

Loons: Wildlife Notebook Series
Loons Loons are known as “spirits of the wilderness,” and it is fitting that Alaska has all five species of loons found in the world. Loons are an integral part of Alaska's wilderness—a living symbol of Alaska's clean water and high level of environmental quality. Loons, especially common loons, are most famous for their call. The cry of a loon piercing the summer twilight is one of the most thrilling sounds of nature. The sight or sound of one of these birds in Alaskan waters gives a special meaning to many, as if it were certifying the surrounding as a truly wild place. Description: Loons have stout bodies, long necks, pointed bills, three-toed webbed feet, and spend most of their time afloat. Loons are sometimes confused with cormorants, mergansers, grebes, and other diving water birds. Loons have solid bones, and compress the air out of their feathers to float low in the water. A loon's bill is held parallel to the water, but the cormorant holds its hooked bill at an angle. Mergansers have narrower bills and a crest. Grebes, also diving water birds, are relatively short-bodied. Loons can be distinguished from ducks in flight by their slower wing beat and low-slung necks and heads. The five species of loons found in Alaska are the common, yellow-billed, red-throated, pacific and arctic. Common loons (Gavia immer), have deep black or dark green heads and necks and dark backs with an intricate pattern of black and white stripes, spots, squares, and rectangles. The yellow-billed loon (Gavia adamsii) is similar, but it has white spots on its back and a straw-yellow bill even in winter. -

Were Sauropod Dinosaurs Responsible for the Warm Mesozoic Climate?
Journal of Palaeogeography 2012, 1(2): 138-148 DOI: 10.3724/SP.J.1261.2012.00011 Biopalaeogeography and palaeoclimatology Were sauropod dinosaurs responsible for the warm Mesozoic climate? A. J. (Tom) van Loon* Geological Institute, Adam Mickiewicz University, Maków Polnych 16, 61-606, Poznan, Poland Abstract It was recently postulated that methane production by the giant Mesozoic sau- ropod dinosaurs was larger than the present-day release of this greenhouse gas by nature and man-induced activities jointly, thus contributing to the warm Mesozoic climate. This conclusion was reached by correct calculations, but these calculations were based on unrealistic as- sumptions: the researchers who postulated this dinosaur-induced warm climate did take into account neither the biomass production required for the sauropods’ food, nor the constraints for the habitats in which the dinosaurs lived, thus neglecting the palaeogeographic conditions. This underlines the importance of palaeogeography for a good understanding of the Earth’s geological history. Key words sauropod dinosaurs, greenhouse conditions, methane, palaeogeography 1 Introduction* etc. Some of these compounds are thought to have contrib‑ uted to the global temperature rise that took place in the For a long time, geologists have wondered why highly 20th century, but the causal relationship is still hotly de‑ significant fluctuations in the global temperature occurred bated (Rothman, 2002). One of the reasons is that climate in the geological past. It was found by Milankovich (1930, models show significant shortcomings (especially when 1936, 1938) that the alternation of Pleistocene glacials and applied back in time). Another reason is that data (e.g. the interglacials can largely be understood on the basis of as‑ relationship between CO2 concentrations in the ice in cores tronomical factors. -

2020 National Bird List
2020 NATIONAL BIRD LIST See General Rules, Eye Protection & other Policies on www.soinc.org as they apply to every event. Kingdom – ANIMALIA Great Blue Heron Ardea herodias ORDER: Charadriiformes Phylum – CHORDATA Snowy Egret Egretta thula Lapwings and Plovers (Charadriidae) Green Heron American Golden-Plover Subphylum – VERTEBRATA Black-crowned Night-heron Killdeer Charadrius vociferus Class - AVES Ibises and Spoonbills Oystercatchers (Haematopodidae) Family Group (Family Name) (Threskiornithidae) American Oystercatcher Common Name [Scientifc name Roseate Spoonbill Platalea ajaja Stilts and Avocets (Recurvirostridae) is in italics] Black-necked Stilt ORDER: Anseriformes ORDER: Suliformes American Avocet Recurvirostra Ducks, Geese, and Swans (Anatidae) Cormorants (Phalacrocoracidae) americana Black-bellied Whistling-duck Double-crested Cormorant Sandpipers, Phalaropes, and Allies Snow Goose Phalacrocorax auritus (Scolopacidae) Canada Goose Branta canadensis Darters (Anhingidae) Spotted Sandpiper Trumpeter Swan Anhinga Anhinga anhinga Ruddy Turnstone Wood Duck Aix sponsa Frigatebirds (Fregatidae) Dunlin Calidris alpina Mallard Anas platyrhynchos Magnifcent Frigatebird Wilson’s Snipe Northern Shoveler American Woodcock Scolopax minor Green-winged Teal ORDER: Ciconiiformes Gulls, Terns, and Skimmers (Laridae) Canvasback Deep-water Waders (Ciconiidae) Laughing Gull Hooded Merganser Wood Stork Ring-billed Gull Herring Gull Larus argentatus ORDER: Galliformes ORDER: Falconiformes Least Tern Sternula antillarum Partridges, Grouse, Turkeys, and -
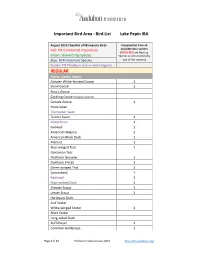
Bird List Lake Pepin IBA REGULAR
Important Bird Area - Bird List Lake Pepin IBA August 2010 Checklist of Minnesota Birds Compiled list from all Red: PIF Continental Importance available data sources (BOLD RED are Nesting Green: Stewardship Species Species as documented by Blue: BCR Important Species one of the sources) Purple: PIF Priority in one or more regions REGULAR Ducks, Geese, Swans Greater White-fronted Goose 1 Snow Goose 1 Ross's Goose Cackling Goose (tallgrass prairie) Canada Goose 1 Mute Swan Trumpeter Swan Tundra Swan 1 Wood Duck 1 Gadwall 1 American Wigeon 1 American Black Duck 1 Mallard 1 Blue-winged Teal 1 Cinnamon Teal Northern Shoveler 1 Northern Pintail 1 Green-winged Teal 1 Canvasback 1 Redhead 1 Ring-necked Duck 1 Greater Scaup 1 Lesser Scaup 1 Harlequin Duck Surf Scoter White-winged Scoter 1 Black Scoter Long-tailed Duck Bufflehead 1 Common Goldeneye 1 Page 1 of 12 Publication date January 2015 http://mn.audubon.org/ Important Bird Area - Bird List Lake Pepin IBA August 2010 Checklist of Minnesota Birds Compiled list from all Red: PIF Continental Importance available data sources (BOLD RED are Nesting Green: Stewardship Species Species as documented by Blue: BCR Important Species one of the sources) Purple: PIF Priority in one or more regions Hooded Merganser 1 Common Merganser 1 Red-breasted Merganser 1 Ruddy Duck 1 Partridge, Grouse, Turkey Gray Partridge 1 Ring-necked Pheasant 1 Ruffed Grouse 1 Spruce Grouse Sharp-tailed Grouse Greater Prairie-Chicken Wild Turkey 1 Loons Red-throated Loon Pacific Loon Common Loon 1 Grebes Pied-billed Grebe 1 Horned -

The Effects of Human Disturbance on Common Loon Productivity In
The effects of human disturbance on common loon productivity in northwestern Montana by Lynn Michelle Kelly A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Fish and Wildlife Management Montana State University © Copyright by Lynn Michelle Kelly (1992) Abstract: Productivity and effects of human disturbance on common loons (Gavia immer)was studied from 1986-1991 in the Tobacco-Stillwater and Clearwater-Swan drainages in northwestern Montana. The adult loon population in these 2 drainages makes up approximately 30% of Montana's population. A density of 72.2 ha of lake surface area per loon was determined. Seventeen pairs exhibited territorial behavior and an average of 10 pairs were successful in raising at least one chick. Ninety percent of nests were located on islands situated in open water, along transitional swamp shorelines or within marshes. Fifty-two percent (n=23) of nests on islands in open water, 64% (n=l1) of nests along transitional swamp shorelines and 75% (n=4) of nests within marshes were successful. Successful nests had a significantly deeper water access than unsuccessful nests. Significant differences in vegetation surrounding nest sites were observed between the 2 drainages. Nest losses with known causes were attributed to flooding, wash-out by wave action, and infertility. Suspected causes of nest failures included human disturbance, dropping water levels and interactions with bald eagles. Reuse of a physiographic area for nesting occurred 94% (n=32) of the time. Nests were located within 50 m of a previously used nest bowl 50% of the time. Loons nesting successfully one year reused the area within 50 m of the previous successful nest over 60% of the time.