Were Sauropod Dinosaurs Responsible for the Warm Mesozoic Climate?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Point Reyes National Seashore Bird List
Birds of Point Reyes National Seashore Gaviidae (Loons) Alcedinidae (Kingfishers) Podicipedidae (Grebes) Picidae (Woodpeckers) Diomedeidae (Albatrosses) Tyrannidae (Tyrant Flycatcher) Procellariidae (Shearwaters, Petrels) Alaudidae (Larks) Hydrobatidae (Storm Petrels) Hirundinidae (Swallows) Sulidae (Boobies, Gannets) Laniidae (Shrikes) Pelecanidae (Pelicans) Vireonidae (Vireos) Phalacrocoracidae (Cormorants) Corvidae (Crows, Jays) Fregatidae (Frigate Birds) Paridae (Chickadees, Titmice) Ardeidae (Herons, Bitterns, & Egrets) Aegithalidae (Bushtits) Threskiornithidae (Ibises, Spoonbills) Sittidae (Nuthatches) Ciconiidae (Storks) Certhiidae (Creepers) Anatidae (Ducks, Geese, Swans) Troglodytidae (Wrens) Cathartidae (New World Vultures) Cinclidae (Dippers) Accipitridae (Hawks, Kites, Eagles) & Regulidae (Kinglets) Falconidae (Caracaras, Falcons) Sylviidae (Old World Warblers, Gnatcatchers) Odontophoridae (New World Quail) Turdidae (Thrushes) Rallidae (Rails, Gallinules, Coots) Timaliidae (Babblers) Gruidae (Cranes) Mimidae (Mockingbirds, Thrashers) Charadriidae (Lapwings, Plovers) Motacillidae (Wagtails, Pipits) Haematopodidae (Oystercatcher) Bombycillidae (Waxwings) Recurvirostridae (Stilts, Avocets) Ptilogonatidae (Silky-flycatcher) Scolopacidae (Sandpipers, Phalaropes) Parulidae (Wood Warblers) Laridae (Skuas, Gulls, Terns, Skimmers) Cardinalidae (Cardinals) Alcidae (Auks, Murres, Puffins) Emberizidae (Emberizids) Columbidae (Pigeons, Doves) Fringillidae (Finches) Cuculidae (Cuckoos, Road Runners, Anis) NON-NATIVES Tytonidae (Barn Owls) -

The Paleontograph______
__________The Paleontograph________ A newsletter for those interested in all aspects of Paleontology Volume 5 Issue 1 March, 2016 _________________________________________________________________ From Your Editor Welcome to our latest issue. I hope you enjoyed the holidays. If you are anything like me, you are looking forward to Spring. We've had a mild winter here in CO. Weather is different than the east coast. While the nights are colder, the days are warmer. It's a nice change for me. I finally have my fossil lab up and running and I am spending my days, or part thereof, working off my backlog of fossils. It has been a couple of months since our last issue but Bob has kept writing and so we have an interesting issue for you to enjoy. The Paleontograph was created in 2012 to continue what was originally the newsletter of The New Jersey Paleontological Society. The Paleontograph publishes articles, book reviews, personal accounts, and anything else that relates to Paleontology and fossils. Feel free to submit both technical and non-technical work. We try to appeal to a wide range of people interested in fossils. Articles about localities, specific types of fossils, fossil preparation, shows or events, museum displays, field trips, websites are all welcome. This newsletter is meant to be one by and for the readers. Issues will come out when there is enough content to fill an issue. I encourage all to submit contributions. It will be interesting, informative and fun to read. It can become whatever the readers and contributors want it to be, so it will be a work in progress. -
![LOONS and GREBES [ ] Common Loon [ ] Pied-Billed Grebe---X](https://docslib.b-cdn.net/cover/1406/loons-and-grebes-common-loon-pied-billed-grebe-x-351406.webp)
LOONS and GREBES [ ] Common Loon [ ] Pied-Billed Grebe---X
LOONS and GREBES [ ] Common Merganser [ ] Common Loon [ ] Ruddy Duck---x OWLS [ ] Pied-billed Grebe---x [ ] Barn Owl [ ] Horned Grebe HAWKS, KITES and EAGLES [ ] Eared Grebe [ ] Northern Harrier SWIFTS and HUMMINGBIRDS [ ] Western Grebe [ ] Cooper’s Hawk [ ] White-throated Swift [ ] Clark’s Grebe [ ] Red-shouldered Hawk [ ] Anna’s Hummingbird---x [ ] Red-tailed Hawk KINGFISHERS PELICANS and CORMORANTS [ ] Golden Eagle [ ] Belted Kingfisher [ ] Brown Pelican [ ] American Kestrel [ ] Double-crested Cormorant [ ] White-tailed Kite WOODPECKERS [ ] Acorn Woodpecker BITTERNS, HERONS and EGRETS PHEASANTS and QUAIL [ ] Red-breasted Sapsucker [ ] American Bittern [ ] Ring-necked Pheasant [ ] Nuttall’s Woodpecker---x [ ] Great Blue Heron [ ] California Quail [ ] Great Egret [ ] Downy Woodpecker [ ] Snowy Egret RAILS [ ] Northern Flicker [ ] Sora [ ] Green Heron---x TYRANT FLYCATCHERS [ ] Black-crowned Night-Heron---x [ ] Common Moorhen Pacific-slope Flycatcher [ ] American Coot---x [ ] NEW WORLD VULTURES [ ] Black Phoebe---x Say’s Phoebe [ ] Turkey Vulture SHOREBIRDS [ ] [ ] Killdeer---x [ ] Ash-throated Flycatcher WATERFOWL [ ] Greater Yellowlegs [ ] Western Kingbird [ ] Greater White-fronted Goose [ ] Black-necked Stilt SHRIKES [ ] Ross’s Goose [ ] Spotted Sandpiper Loggerhead Shrike [ ] Canada Goose---x [ ] Least Sandpiper [ ] [ ] Wood Duck [ ] Long-billed Dowitcher VIREOS Gadwall [ ] [ ] Wilson’s Snipe [ ] Warbling Vireo [ ] American Wigeon [ ] Mallard---x GULLS and TERNS JAYS and CROWS [ ] Cinnamon Teal [ ] Mew Gull [ ] Western Scrub-Jay---x -

Postcranial Skeletal Pneumaticity in Sauropods and Its
Postcranial Pneumaticity in Dinosaurs and the Origin of the Avian Lung by Mathew John Wedel B.S. (University of Oklahoma) 1997 A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Integrative Biology in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Kevin Padian, Co-chair Professor William Clemens, Co-chair Professor Marvalee Wake Professor David Wake Professor John Gerhart Spring 2007 1 The dissertation of Mathew John Wedel is approved: Co-chair Date Co-chair Date Date Date Date University of California, Berkeley Spring 2007 2 Postcranial Pneumaticity in Dinosaurs and the Origin of the Avian Lung © 2007 by Mathew John Wedel 3 Abstract Postcranial Pneumaticity in Dinosaurs and the Origin of the Avian Lung by Mathew John Wedel Doctor of Philosophy in Integrative Biology University of California, Berkeley Professor Kevin Padian, Co-chair Professor William Clemens, Co-chair Among extant vertebrates, postcranial skeletal pneumaticity is present only in birds. In birds, diverticula of the lungs and air sacs pneumatize specific regions of the postcranial skeleton. The relationships among pulmonary components and the regions of the skeleton that they pneumatize form the basis for inferences about the pulmonary anatomy of non-avian dinosaurs. Fossae, foramina and chambers in the postcranial skeletons of pterosaurs and saurischian dinosaurs are diagnostic for pneumaticity. In basal saurischians only the cervical skeleton is pneumatized. Pneumatization by cervical air sacs is the most consilient explanation for this pattern. In more derived sauropods and theropods pneumatization of the posterior dorsal, sacral, and caudal vertebrae indicates that abdominal air sacs were also present. -

Common Birds of the Estero Bay Area
Common Birds of the Estero Bay Area Jeremy Beaulieu Lisa Andreano Michael Walgren Introduction The following is a guide to the common birds of the Estero Bay Area. Brief descriptions are provided as well as active months and status listings. Photos are primarily courtesy of Greg Smith. Species are arranged by family according to the Sibley Guide to Birds (2000). Gaviidae Red-throated Loon Gavia stellata Occurrence: Common Active Months: November-April Federal Status: None State/Audubon Status: None Description: A small loon seldom seen far from salt water. In the non-breeding season they have a grey face and red throat. They have a long slender dark bill and white speckling on their dark back. Information: These birds are winter residents to the Central Coast. Wintering Red- throated Loons can gather in large numbers in Morro Bay if food is abundant. They are common on salt water of all depths but frequently forage in shallow bays and estuaries rather than far out at sea. Because their legs are located so far back, loons have difficulty walking on land and are rarely found far from water. Most loons must paddle furiously across the surface of the water before becoming airborne, but these small loons can practically spring directly into the air from land, a useful ability on its artic tundra breeding grounds. Pacific Loon Gavia pacifica Occurrence: Common Active Months: November-April Federal Status: None State/Audubon Status: None Description: The Pacific Loon has a shorter neck than the Red-throated Loon. The bill is very straight and the head is very smoothly rounded. -

Final Restoration Plan for Common Loon and Other Birds Impacted by the Bouchard Barge 120 (B-120) Oil Spill, Buzzards Bay Massachusetts and Rhode Island
FINAL RESTORATION PLAN for COMMON LOON (Gavia immer) and OTHER BIRDS IMPACTED BY THE BOUCHARD BARGE 120 (B-120) OIL SPILL BUZZARDS BAY MASSACHUSETTS and RHODE ISLAND June 2020 Prepared by: United States Fish and Wildlife Service Massachusetts Executive Office of Energy and Environmental Affairs Rhode Island Department of Environmental Management and National Oceanic and Atmospheric Administration (Lead Administrative Trustee) Executive Summary In April 2003, the Bouchard Barge‐120 (B‐120) oil spill (the Spill) affected more than 100 miles of Buzzards Bay and its shoreline and nearby coastal waters in both Massachusetts (MA) and Rhode Island (RI). Birds were exposed to and ingested oil as they foraged, nested, and/or migrated through the area. Species of birds estimated to have been killed in the greatest numbers included common loon (Gavia immer), common and roseate terns (Sterna hirundo and Sterna dougallii), and other birds such as common eider (Somateria mollissima), black scoter (Melanitta americana), and red‐throated loon (Gavia stellata). The National Oceanic and Atmospheric Administration (NOAA), U.S. Department of the Interior (DOI) (acting through the U.S. Fish and Wildlife Service [USFWS]), the Commonwealth of Massachusetts (acting through the Executive Office of Energy and Environmental Affairs [EEA]), and the State of Rhode Island serve as the natural resource Trustees (Trustees) responsible under the Oil Pollution Act of 1990 (OPA) (33 U.S.C. § 2701, et seq.) for ensuring the natural resources injured from the Spill are restored. As a designated Trustee, each agency is authorized to act on behalf of the public under State1 and/or Federal law to assess and recover natural resource damages, and to plan and implement actions to restore, rehabilitate, replace, or acquire the equivalent of the natural resources or services injured or lost as a result of an unpermitted discharge of oil. -

Back Matter (PDF)
Index Note: Page numbers in italic denote figures. Page numbers in bold denote tables. Abel, Othenio (1875–1946) Ashmolean Museum, Oxford, Robert Plot 7 arboreal theory 244 Astrodon 363, 365 Geschichte und Methode der Rekonstruktion... Atlantosaurus 365, 366 (1925) 328–329, 330 Augusta, Josef (1903–1968) 222–223, 331 Action comic 343 Aulocetus sammarinensis 80 Actualism, work of Capellini 82, 87 Azara, Don Felix de (1746–1821) 34, 40–41 Aepisaurus 363 Azhdarchidae 318, 319 Agassiz, Louis (1807–1873) 80, 81 Azhdarcho 319 Agustinia 380 Alexander, Annie Montague (1867–1950) 142–143, 143, Bakker, Robert. T. 145, 146 ‘dinosaur renaissance’ 375–376, 377 Alf, Karen (1954–2000), illustrator 139–140 Dinosaurian monophyly 93, 246 Algoasaurus 365 influence on graphic art 335, 343, 350 Allosaurus, digits 267, 271, 273 Bara Simla, dinosaur discoveries 164, 166–169 Allosaurus fragilis 85 Baryonyx walkeri Altispinax, pneumaticity 230–231 relation to Spinosaurus 175, 177–178, 178, 181, 183 Alum Shale Member, Parapsicephalus purdoni 195 work of Charig 94, 95, 102, 103 Amargasaurus 380 Beasley, Henry Charles (1836–1919) Amphicoelias 365, 366, 368, 370 Chirotherium 214–215, 219 amphisbaenians, work of Charig 95 environment 219–220 anatomy, comparative 23 Beaux, E. Cecilia (1855–1942), illustrator 138, 139, 146 Andrews, Roy Chapman (1884–1960) 69, 122 Becklespinax altispinax, pneumaticity 230–231, Andrews, Yvette 122 232, 363 Anning, Joseph (1796–1849) 14 belemnites, Oxford Clay Formation, Peterborough Anning, Mary (1799–1847) 24, 25, 113–116, 114, brick pits 53 145, 146, 147, 288 Benett, Etheldred (1776–1845) 117, 146 Dimorphodon macronyx 14, 115, 294 Bhattacharji, Durgansankar 166 Hawker’s ‘Crocodile’ 14 Birch, Lt. -

Beached Bird Guide for Northern Lake Michigan
Beached Bird Guide for Northern Lake Michigan Prepared by Common Coast Research & Conservation In association with the Grand Traverse Bay Botulism Network © 2008 Common Coast Research & Conservation How to use this guide This guide was developed to aid with the field identification of the most common waterbird species implicated in botulism E die-offs on northern Lake Michigan. The guide is not intended to be a comprehensive treatment of all species you may encounter in the field. For birds not treated in this guide please document with photographs and/or submit carcasses to the nearest Michigan Department of Natural Resources Field Office for identification and/or testing for botulism (see manual). The emphasis of this guide is on differences in bill structure among the various waterbird species. The bill plates are drawn to actual size - we recommend laminating the guide for use in the field. Placing the bills of unknown species directly on the plates will facilitate identification. Please keep in mind some variation among individuals is to be expected. Photographs of unknown species are helpful for later identification. Bird Topography tarsus crown bill (upper and lower mandibles) foot bill margin cheek throat wing coverts (lesser) secondaries webbed foot lobed foot primaries (loons, ducks, gulls) (grebes) Loons and Grebes Birds with dagger-like bills Description: Adult Common Loon bill large, dagger-like, mandible edges smooth feet webbed tarsus narrow, flat Plumage variation (adult vs. juvenile): Look at wing coverts: Adult – well-defined white "windows" (see photo) Juvenile - lacks defined white "windows" Similar species: Red-throated Loon – bill smaller (rarely found) Red-necked Grebe – feet lobed, bill smaller Description: Red-throated Loon bill dagger-like, slightly upturned, mandible edges smooth feet webbed tarsus narrow, flat Similar species: Common Loon - larger; bill heavier, not upturned Red-necked Grebe – feet lobed , bill yellowish NOTE: Rarely encountered. -

A New Giant Basal Titanosaur Sauropod in the Upper Cretaceous (Coniacian) of the Neuquen� Basin, Argentina
Cretaceous Research 100 (2019) 61e81 Contents lists available at ScienceDirect Cretaceous Research journal homepage: www.elsevier.com/locate/CretRes A new giant basal titanosaur sauropod in the Upper Cretaceous (Coniacian) of the Neuquen Basin, Argentina * Leonardo S. Filippi a, , Leonardo Salgado b, c, Alberto C. Garrido d, e a Museo Municipal Argentino Urquiza, Jujuy y Chaco s/n, 8319 Rincon de los Sauces, Neuquen, Argentina b CONICET, Argentina c Instituto de Investigacion en Paleobiología y Geología, Universidad Nacional de Río Negro-Conicet, Av. Gral. J. A. Roca 1242, 8332 General Roca, Río Negro, Argentina d Museo Provincial de Ciencias Naturales “Profesor Dr. Juan A. Olsacher”, Direccion Provincial de Minería, Etcheluz y Ejercito Argentino, 8340 Zapala, Neuquen, Argentina e Departamento Geología y Petroleo, Facultad de Ingeniería, Universidad Nacional del Comahue, Buenos Aires 1400, Neuquen 8300, provincia del Neuquen, Argentina article info abstract Article history: A new basal sauropod titanosaur, Kaijutitan maui gen. et sp. nov., is described. The holotype of this Received 21 November 2018 species, which comes from the Sierra Barrosa Formation (upper Coniacian, Upper Cretaceous), consists of Received in revised form cranial, axial, and appendicular elements presenting an unique combination of plesiomorphic and 3 February 2019 apomorphic characters. The most notable characteristic observed in Kaijutitan is the presence of anterior Accepted in revised form 9 March 2019 cervical vertebrae with bifid neural spines, a condition that would have evolved several times among Available online 28 March 2019 sauropods. The phylogenetic analysis places Kaijutitan as a basal titanosaur, the sister taxon of Epachthosaurus þ Eutitanosauria. The new species supports the coexistence, in the Late Cretaceous Keywords: Sauropoda (Turonian-Santonian), of basal titanosaurs and eutitanosaurian sauropods, at least in Patagonia. -

Loons: Wildlife Notebook Series
Loons Loons are known as “spirits of the wilderness,” and it is fitting that Alaska has all five species of loons found in the world. Loons are an integral part of Alaska's wilderness—a living symbol of Alaska's clean water and high level of environmental quality. Loons, especially common loons, are most famous for their call. The cry of a loon piercing the summer twilight is one of the most thrilling sounds of nature. The sight or sound of one of these birds in Alaskan waters gives a special meaning to many, as if it were certifying the surrounding as a truly wild place. Description: Loons have stout bodies, long necks, pointed bills, three-toed webbed feet, and spend most of their time afloat. Loons are sometimes confused with cormorants, mergansers, grebes, and other diving water birds. Loons have solid bones, and compress the air out of their feathers to float low in the water. A loon's bill is held parallel to the water, but the cormorant holds its hooked bill at an angle. Mergansers have narrower bills and a crest. Grebes, also diving water birds, are relatively short-bodied. Loons can be distinguished from ducks in flight by their slower wing beat and low-slung necks and heads. The five species of loons found in Alaska are the common, yellow-billed, red-throated, pacific and arctic. Common loons (Gavia immer), have deep black or dark green heads and necks and dark backs with an intricate pattern of black and white stripes, spots, squares, and rectangles. The yellow-billed loon (Gavia adamsii) is similar, but it has white spots on its back and a straw-yellow bill even in winter. -
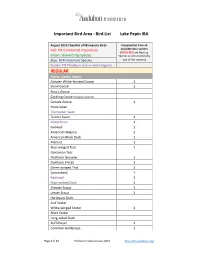
Bird List Lake Pepin IBA REGULAR
Important Bird Area - Bird List Lake Pepin IBA August 2010 Checklist of Minnesota Birds Compiled list from all Red: PIF Continental Importance available data sources (BOLD RED are Nesting Green: Stewardship Species Species as documented by Blue: BCR Important Species one of the sources) Purple: PIF Priority in one or more regions REGULAR Ducks, Geese, Swans Greater White-fronted Goose 1 Snow Goose 1 Ross's Goose Cackling Goose (tallgrass prairie) Canada Goose 1 Mute Swan Trumpeter Swan Tundra Swan 1 Wood Duck 1 Gadwall 1 American Wigeon 1 American Black Duck 1 Mallard 1 Blue-winged Teal 1 Cinnamon Teal Northern Shoveler 1 Northern Pintail 1 Green-winged Teal 1 Canvasback 1 Redhead 1 Ring-necked Duck 1 Greater Scaup 1 Lesser Scaup 1 Harlequin Duck Surf Scoter White-winged Scoter 1 Black Scoter Long-tailed Duck Bufflehead 1 Common Goldeneye 1 Page 1 of 12 Publication date January 2015 http://mn.audubon.org/ Important Bird Area - Bird List Lake Pepin IBA August 2010 Checklist of Minnesota Birds Compiled list from all Red: PIF Continental Importance available data sources (BOLD RED are Nesting Green: Stewardship Species Species as documented by Blue: BCR Important Species one of the sources) Purple: PIF Priority in one or more regions Hooded Merganser 1 Common Merganser 1 Red-breasted Merganser 1 Ruddy Duck 1 Partridge, Grouse, Turkey Gray Partridge 1 Ring-necked Pheasant 1 Ruffed Grouse 1 Spruce Grouse Sharp-tailed Grouse Greater Prairie-Chicken Wild Turkey 1 Loons Red-throated Loon Pacific Loon Common Loon 1 Grebes Pied-billed Grebe 1 Horned -

Osteology of the Dorsal Vertebrae of the Giant Titanosaurian Sauropod Dinosaur Dreadnoughtus Schrani from the Late Cretaceous of Argentina
Editors' choice Osteology of the dorsal vertebrae of the giant titanosaurian sauropod dinosaur Dreadnoughtus schrani from the Late Cretaceous of Argentina KRISTYN K. VOEGELE, MATTHEW C. LAMANNA, and KENNETH J. LACOVARA Voegele, K.K., Lamanna, M.C., and Lacovara K.J. 2017. Osteology of the dorsal vertebrae of the giant titanosaurian sauropod dinosaur Dreadnoughtus schrani from the Late Cretaceous of Argentina. Acta Palaeontologica Polonica 62 (4): 667–681. Many titanosaurian dinosaurs are known only from fragmentary remains, making comparisons between taxa difficult because they often lack overlapping skeletal elements. This problem is particularly pronounced for the exceptionally large-bodied members of this sauropod clade. Dreadnoughtus schrani is a well-preserved giant titanosaurian from the Upper Cretaceous (Campanian–Maastrichtian) Cerro Fortaleza Formation of southern Patagonia, Argentina. Numerous skeletal elements are known for Dreadnoughtus, including seven nearly complete dorsal vertebrae and a partial dorsal neural arch that collectively represent most of the dorsal sequence. Here we build on our previous preliminary descrip- tion of these skeletal elements by providing a detailed assessment of their serial positional assignments, as well as comparisons of the dorsal vertebrae of Dreadnoughtus with those of other exceptionally large-bodied titanosaurians. Although the dorsal elements of Dreadnoughtus probably belong to two individuals, they exhibit substantial morpho- logical variation that suggests that there is minimal, if any, positional overlap among them. Dreadnoughtus therefore preserves the second-most complete dorsal vertebral series known for a giant titanosaurian that has been described in detail, behind only that of Futalognkosaurus. The dorsal sequence of Dreadnoughtus provides valuable insight into serial variation along the vertebral column of these enormous sauropods.