A Pattern of Unique Embryogenesis Occurring Via Apomixis in Carya Cathayensis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Topic: Apomixis - Definition and Types
Course Teacher: Dr. Rupendra Kumar Jhade, JNKVV, College of Horticulture,Chhindwara(M.P.) B.Sc.(Hons) Horticulture- 1st Year, II Semester 2019-20 Course: Plant Propagation and Nursery Management Lecture No. 12 Topic: Apomixis - Definition and types Apomixis, derived from two Greek word “APO” (away from) and “MIXIS” (act of mixing or mingling). The first discovery of this phenomenon is credited to Leuwen hock as early as 1719 in Citrus seeds. In apomixes, seeds are formed but the embryos develop without fertilization. The genotype of the embryo and resulting plant will be the same as the seed parent. Definition: “The process of development of diploid embryos without fertilization.” Or “Apomixis is a form of asexual reproduction that occurs via seeds, in which embryos develop without fertilization.” Or “Apomixis is a type of reproduction in which sexual organs of related structures take part but seeds are formed without union of gametes.” In some species of plants, an embryo develops from the diploid cells of the seed and not as a result of fertilization between ovule and pollen. This type of reproduction is known as apomixis and the seedlings produced in this manner are known as apomicts. Apomictic seedlings are identical to mother plant and similar to plants raised by other vegetative means, because such plants have the same genetic make-up as that of the mother plant. Such seedlings are completely free from viruses. In apomictic species, sexual reproduction is either suppressed or absent. When sexual reproduction also occurs, the apomixes is termed as facultative apomixis. But when sexual reproduction is absent, it is referred to as obligate apomixis. -

Tropical Horticulture: Lecture 32 1
Tropical Horticulture: Lecture 32 Lecture 32 Citrus Citrus: Citrus spp., Rutaceae Citrus are subtropical, evergreen plants originating in southeast Asia and the Malay archipelago but the precise origins are obscure. There are about 1600 species in the subfamily Aurantioideae. The tribe Citreae has 13 genera, most of which are graft and cross compatible with the genus Citrus. There are some tropical species (pomelo). All Citrus combined are the most important fruit crop next to grape. 1 Tropical Horticulture: Lecture 32 The common features are a superior ovary on a raised disc, transparent (pellucid) dots on leaves, and the presence of aromatic oils in leaves and fruits. Citrus has increased in importance in the United States with the development of frozen concentrate which is much superior to canned citrus juice. Per-capita consumption in the US is extremely high. Citrus mitis (calamondin), a miniature orange, is widely grown as an ornamental house pot plant. History Citrus is first mentioned in Chinese literature in 2200 BCE. First citrus in Europe seems to have been the citron, a fruit which has religious significance in Jewish festivals. Mentioned in 310 BCE by Theophrastus. Lemons and limes and sour orange may have been mutations of the citron. The Romans grew sour orange and lemons in 50–100 CE; the first mention of sweet orange in Europe was made in 1400. Columbus brought citrus on his second voyage in 1493 and the first plantation started in Haiti. In 1565 the first citrus was brought to the US in Saint Augustine. 2 Tropical Horticulture: Lecture 32 Taxonomy Citrus classification based on morphology of mature fruit (e.g. -

Morphological and Genetic Analysis of Embryo Specific Mutants in Maize
University of North Dakota UND Scholarly Commons Theses and Dissertations Theses, Dissertations, and Senior Projects January 2018 Morphological And Genetic Analysis Of Embryo Specific utM ants In Maize Dale Cletus Brunelle Follow this and additional works at: https://commons.und.edu/theses Recommended Citation Brunelle, Dale Cletus, "Morphological And Genetic Analysis Of Embryo Specific utM ants In Maize" (2018). Theses and Dissertations. 2392. https://commons.und.edu/theses/2392 This Dissertation is brought to you for free and open access by the Theses, Dissertations, and Senior Projects at UND Scholarly Commons. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of UND Scholarly Commons. For more information, please contact [email protected]. MORPHOLOGICAL AND GENETIC ANALYSIS OF EMBRYO SPECIFIC MUTANTS IN MAIZE by Dale Cletus Brunelle Bachelor of Science, University of North Dakota, 2009 Master of Science, University of North Dakota, 2011 A Dissertation Submitted to the Graduate Faculty of the University of North Dakota in partial fulfillment of the requirements for the degree of Doctor of Philosophy Grand Forks, North Dakota December 2018 i PERMISSION Title- Morphological and Genetic Analysis of Embryo Specific Mutants in Maize Department Biology Degree Doctor of Philosophy In presenting this dissertation in partial fulfillment of the requirements for a graduate degree from the University of North Dakota, I agree that the library of this University shall make it freely available for inspection. I further agree that permission for extensive copying for scholarly purposes may be granted by the professor who supervised my dissertation work or, in his absence, by the Chairperson of the department or the dean of the School of Graduate Studies. -

Comparative Transcriptome Sequencing to Determine Genes Related to the Nucellar Embryony Mechanism in Citrus
Turkish Journal of Agriculture and Forestry Turk J Agric For (2019) 43: 58-68 http://journals.tubitak.gov.tr/agriculture/ © TÜBİTAK Research Article doi:10.3906/tar-1806-10 Comparative transcriptome sequencing to determine genes related to the nucellar embryony mechanism in citrus 1, 2 1 1 1 Özhan ŞİMŞEK *, Dicle DÖNMEZ , Sinan ETİ , Turgut YEŞİLOĞLU , Yıldız AKA KAÇAR 1 Department of Horticulture, Faculty of Agriculture, Çukurova University, Adana, Turkey 2 Biotechnology Research and Application Center, Çukurova University, Adana, Turkey Received: 04.06.2018 Accepted/Published Online: 14.10.2018 Final Version: 06.02.2019 Abstract: Several citrus varieties produce apomictic seeds by the nucellar embryony (NE) mechanism. Nucellar embryos are created from nucellus tissue and have an identical genetic constitution to the mother plant. Nucellar embryony is known to be an unusual feature of seed production in many citrus cultivars. The term “NE” refers to the development of identical embryos from the maternal tissue known as the nucellus surrounding the embryo sac. The authors aimed here to detect differentially expressed genes involved in the NE mechanism. Orlando tangelo (OT), producing apomictic seeds, and a clementine mandarin, Algerian tangerine ranch selection (AT), known as monoembryonic, were used for high-throughput transcriptome sequencing. First of all, histological analysis was used to determine the initial stage of the development of NE cells. Initial NE cells began to develop on the third day after anthesis. Based on the histological analysis, ovules of flower buds for OT were sampled at the balloon stage and 1, 3, and 5 days after anthesis; for AT only ovules of flower buds at the balloon stage and 3 days after anthesis were sampled for comparative transcriptome sequencing. -

Development of the Embryo and the Young Seedling Stages of Orchids
Development of the Embryo II' L_ and the Young Seedling Stages of Orchids YVONNE VEYRET Until 1804, when Salisbury found that orchid seeds could germinate, the seeds were considered sterile. Much later, in 1889, Bernard's discovery of the special conditions necessary for their growth and development ex- plained the lack of success in previous attempts to obtain plantules, and the success, although mediocre, when sowing of seed took place at the foot of the mother plant. Knowing the rudimentary state of minute or- chid embryos, one can better understand that an exterior agent, usually a Rhizoctonia, can be useful in helping them through their first stages of development (Bernard, 1904). Orchid embryos, despite their rudimentary condition, present diverse pattems of development, the most apparent of which are concerned with the character of the suspensor; there are other basic patterns that are re- vealed in the course of the formation of the embryonic body. These char- acteristics have been used differently in the classification of orchid em- bryos. This will be discussed in the first part of this chapter, to which will be added our knowledge of other phenomena concerning the embryo, in particular polyembryonic and apomictic seed formation. The second part of this chapter is devoted to the development of the young embryo, a study necessary for understanding the evolution of the different zones of the embryonic mass. We will also examine the characteristics of the em- bryo whose morphology, biology, and development are specialized when compared to other plants. 3 /-y , I', 1 dT 1- , ,)i L 223 - * * C" :. -

Improvement of Subtropical Fruit Crops: Citrus
IMPROVEMENT OF SUBTROPICAL FRUIT CROPS: CITRUS HAMILTON P. ÏRAUB, Senior Iloriiciilturist T. RALPH ROBCNSON, Senior Physiolo- gist Division of Frnil and Vegetable Crops and Diseases, Bureau of Plant Tndusiry MORE than half of the 13 fruit crops known to have been cultivated longer than 4,000 years,according to the researches of DeCandolle (7)\ are tropical and subtropical fruits—mango, oliv^e, fig, date, banana, jujube, and pomegranate. The citrus fruits as a group, the lychee, and the persimmon have been cultivated for thousands of years in the Orient; the avocado and papaya were important food crops in the American Tropics and subtropics long before the discovery of the New World. Other types, such as the pineapple, granadilla, cherimoya, jaboticaba, etc., are of more recent introduction, and some of these have not received the attention of the plant breeder to any appreciable extent. Through the centuries preceding recorded history and up to recent times, progress in the improvement of most subtropical fruits was accomplished by the trial-error method, which is crude and usually expensive if measured by modern standards. With the general accept- ance of the Mendelian principles of heredity—unit characters, domi- nance, and segregation—early in the twentieth century a starting point was provided for the development of a truly modern science of genetics. In this article it is the purpose to consider how subtropical citrus fruit crops have been improved, are now being improved, or are likel3^ to be improved by scientific breeding. Each of the more important crops will be considered more or less in detail. -

Citrus Rootstocks: Their Characters and Reactions
CITRUS ROOTSTOCKS: THEIR CHARACTERS AND REACTIONS (an unpublished manuscript) ca. 1986 By W. P. BITTERS (1915 – 2006) Editor, digital version: Marty Nemeth, Reference Librarian, UC Riverside Science Library, retired Subject matter experts, digital version: Dr. Tracy Kahn, Curator, UC Citrus Variety Collection Dr. Robert Krueger, Curator, USDA-ARS National Clonal Germplasm Repository for Citrus & Dates Toni Siebert, Assistant Curator, UC Citrus Variety Collection ca. 1955 ca. 1970 IN MEMORIUM Willard P. Bitters Professor of Horticulture, Emeritus Riverside 1915-2006 Born in Eau Claire, Wisconsin, in June, 1915, Dr. Willard “Bill” Bitters earned his bachelor’s degree in biology from St. Norbert College and his master’s degree and Ph.D. from the University of Wisconsin. After earning his doctorate, he first worked as the superintendent of the Valley Research Farm of the University of Arizona in Yuma, and joined the Citrus Experiment Station, in Riverside in 1946 as a Horticulturist. In 1961, Dr. Bitters became a Professor in the newly established University of California-Riverside. His initial assignment was to work on horticultural aspects of tristeza, a serious vector-transmitted virus disease which threatened to destroy California citrus orchards. Tristeza was already in California and spreading in 1946. At that time most citrus trees in California were grafted on a rootstock that was known to be susceptible to tristeza. Dr. Bill Bitters was responsible for screening of over 500 cultivars to determine which rootstock-scion combinations were resistant to this disease and yet possessed suitable horticultural characteristics. Of the 500 screened, most were susceptible, but several successful ones were selected and released to the industry. -

Genomic Analyses of Primitive, Wild and Cultivated Citrus Provide Insights Into Asexual Reproduction
ARTICLES OPEN Genomic analyses of primitive, wild and cultivated citrus provide insights into asexual reproduction Xia Wang1,7, Yuantao Xu1,7, Siqi Zhang1,7, Li Cao2,7, Yue Huang1, Junfeng Cheng3, Guizhi Wu1, Shilin Tian4, Chunli Chen5, Yan Liu3, Huiwen Yu1, Xiaoming Yang1, Hong Lan1, Nan Wang1, Lun Wang1, Jidi Xu1, Xiaolin Jiang1, Zongzhou Xie1, Meilian Tan1, Robert M Larkin1, Ling-Ling Chen3, Bin-Guang Ma3, Yijun Ruan5,6, Xiuxin Deng1 & Qiang Xu1 The emergence of apomixis—the transition from sexual to asexual reproduction—is a prominent feature of modern citrus. Here we de novo sequenced and comprehensively studied the genomes of four representative citrus species. Additionally, we sequenced 100 accessions of primitive, wild and cultivated citrus. Comparative population analysis suggested that genomic regions harboring energy- and reproduction-associated genes are probably under selection in cultivated citrus. We also narrowed the genetic locus responsible for citrus polyembryony, a form of apomixis, to an 80-kb region containing 11 candidate genes. One of these, CitRWP, is expressed at higher levels in ovules of polyembryonic cultivars. We found a miniature inverted-repeat transposable element insertion in the promoter region of CitRWP that cosegregated with polyembryony. This study provides new insights into citrus apomixis and constitutes a promising resource for the mining of agriculturally important genes. Asexual reproduction is a remarkable feature of perennial fruit crops important trait for breeding purposes. On the one hand, polyembryony that facilitates the faithful propagation of commercially valuable is widely employed in citrus nurseries and propagation programs to individuals by avoiding the uncertainty associated with the sexual generate large numbers of uniform rootstocks from seeds. -

UC Riverside UC Riverside Previously Published Works
UC Riverside UC Riverside Previously Published Works Title AFLP markers closely linked to a major gene essential for nucellar embryony (apomixis) in Citrus maxima × Poncirus trifoliata Permalink https://escholarship.org/uc/item/6dh1s48s Journal Tree Genetics & Genomes, 6(1) ISSN 1614-2950 Authors Kepiro, J. L. Roose, M. L. Publication Date 2009-12-01 DOI 10.1007/s11295-009-0223-z Peer reviewed eScholarship.org Powered by the California Digital Library University of California Tree Genetics & Genomes (2010) 6:1–11 DOI 10.1007/s11295-009-0223-z ORIGINAL PAPER AFLP markers closely linked to a major gene essential for nucellar embryony (apomixis) in Citrus maxima × Poncirus trifoliata J. L. Kepiro & M. L. Roose Received: 27 July 2007 /Revised: 29 August 2008 /Accepted: 24 June 2009 /Published online: 28 July 2009 # The Author(s) 2009. This article is published with open access at Springerlink.com Abstract Some citrus varieties express a form of apo- these markers very likely contains a gene that is essential mixis termed nucellar embryony in which the adventive for the production of polyembryonic seeds by apomixis, but embryos develop from nucellus tissue surrounding the also shows segregation distortion. The proportion of embryo sac. This trait results in many seeds containing polyembryonic seeds varied widely among the hybrid multiple embryos (polyembryony). Inheritance of the progeny, probably due to other genes. Scoring 119 progeny frequency of polyembryony was studied in 88 progeny of a P. trifoliata selfed population for the closely linked from a cross of Citrus maxima (monoembryonic) × markers and the proportion of polyembryonic seeds Poncirus trifoliata (polyembryonic). -
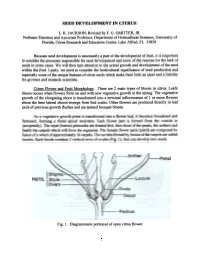
Seed Development in Citrus
SEED DEVELOPMENT IN CITRUS L. K. JACKSON; Revisedby F. G. GMITTER, JR. ProfessorEmeritus and AssociateProfessor, Department of Horticultural Sciences,University of Florida, Citrus Researchand EducationCenter, Lake Alfred, FL 33850 Becauseseed development is necessarilya part of the developmentof fruit. it is important to considerthe processesresponsible for seeddevelopment and someof the reasonsfor the lack of seedsin somecases. We will then turn attentionto the actual growth and developmentof the seed within the fruit. Lastly, we needto considerthe horticultural significanceof seedproduction and especiallysome of the uniquefeatures of citrus seedswhich makethem both an assetand a liability for growersand researchscientists. Citrus Flower and Fruit MoQ1holo~. There are 2 main types of bloom in citrus. Leafy bloom occurswhen flowers form on and with new vegetativegrowth in the spring. The vegetative growth of the elongating shoot is transformedinto a tenninal inflorescenceof 1 or more flowers about the time lateral shootsemerge from bud scales.Other flowers are produceddirectly in leaf axils of previous growth flushesand are termedbouquet bloom. As a vegetative growth point is transfonned into a flower bud, it becomes broadened and flattened, forming a floral apical meristem. Each flower part is fonned from the outside in (acropetally). The sepal (button) primordia are fonned first, then those of the petals, the anthers and finally the carpels which will form the segments. The female flower parts (pistil) are composed by fusion of a whorl of approximately 10 carpels. The cavities fonned by fusion of the carpels are called locules. Each locule contains 2 vertical rows of ovules (Fig. 1), that can develop into seeds. Fig. 1. Diagrammaticportrayal of opencitrus flower. -

12.2% 122,000 135M Top 1% 154 4,800
We are IntechOpen, the world’s leading publisher of Open Access books Built by scientists, for scientists 4,800 122,000 135M Open access books available International authors and editors Downloads Our authors are among the 154 TOP 1% 12.2% Countries delivered to most cited scientists Contributors from top 500 universities Selection of our books indexed in the Book Citation Index in Web of Science™ Core Collection (BKCI) Interested in publishing with us? Contact [email protected] Numbers displayed above are based on latest data collected. For more information visit www.intechopen.com Chapter 2 Polyembryony in Maize: A Complex, Elusive, and Potentially Agronomical Useful Trait Mariela R. Michel, Marisol Cruz-Requena, MarielaMarselino R. Michel,C. Avendaño-Sanchez, Marisol Cruz-Requena, MarselinoVíctor M. González-Vazquez, C. Avendaño-Sanchez, VíctorAdriana M. C. González-Vazquez, Flores-Gallegos, AdrianaCristóbal C. N. Flores-Gallegos, Aguilar, José Espinoza-Velázquez Cristóbal N. Aguilar, and Raúl Rodríguez-Herrera José Espinoza-Velázquez and RaúlAdditional Rodríguez-Herrera information is available at the end of the chapter Additional information is available at the end of the chapter http://dx.doi.org/10.5772/intechopen.70549 Abstract Polyembryony (PE) is a rare phenomenon in cultivated plant species. Since nineteenth cen- tury, several reports have been published on PE in maize. Reports of multiple seedlings developing at embryonic level in laboratory and studies under greenhouse and field condi- tions have demonstrated the presence of PE in cultivated maize (Zea mays L.). Nevertheless, there is a lack of knowledge about this phenomenon; diverse genetic mechanisms controlling PE in maize have been proposed: Mendelian inheritance of a single gene, interaction between two genes and multiple genes are some of the proposed mechanisms. -

Citrus Genetic Resources in California
Citrus Genetic Resources in California Analysis and Recommendations for Long-Term Conservation Report of the Citrus Genetic Resources Assessment Task Force T.L. Kahn, R.R. Krueger, D.J. Gumpf, M.L. Roose, M.L. Arpaia, T.A. Batkin, J.A. Bash, O.J. Bier, M.T. Clegg, S.T. Cockerham, C.W. Coggins Jr., D. Durling, G. Elliott, P.A. Mauk, P.E. McGuire, C. Orman, C.O. Qualset, P.A. Roberts, R.K. Soost, J. Turco, S.G. Van Gundy, and B. Zuckerman Report No. 22 June 2001 Published by Genetic Resources Conservation Program Division of Agriculture and Natural Resources UNIVERSITY OF CALIFORNIA i This report is one of a series published by the University of California Genetic Resources Conservation Program (technical editor: P.E. McGuire) as part of the public information function of the Program. The Program sponsors projects in the collection, inventory, maintenance, preservation, and utilization of genetic resources important for the State of California as well as research and education in conservation biology. Further information about the Program may be obtained from: Genetic Resources Conservation Program University of California One Shields Avenue Davis, CA 95616 USA (530) 754-8501 FAX (530) 754-8505 e-mail: [email protected] Website: http://www.grcp.ucdavis.edu/ Additional copies of this report may be ordered from this address. Citation: Kahn TL, RR Krueger, DJ Gumpf, ML Roose, ML Arpaia, TA Batkin, JA Bash, OJ Bier, MT Clegg, ST Cockerham, CW Coggins Jr, D Durling, G Elliott, PA Mauk, PE McGuire, C Orman, CO Qualset, PA Roberts, RK Soost, J Turco, SG Van Gundy, and B Zuckerman.