Keratinocyte-Specific Ablation of the NF-&Kappa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hair Histology As a Tool for Forensic Identification of Some Domestic Animal Species
EXCLI Journal 2018;17:663-670 – ISSN 1611-2156 Received: June 28, 2018, accepted: July 02, 2018, published: July 06, 2018 Original article: HAIR HISTOLOGY AS A TOOL FOR FORENSIC IDENTIFICATION OF SOME DOMESTIC ANIMAL SPECIES Yasser A. Ahmed1, Safwat Ali2, Ahmed Ghallab3* 1 Department of Histology, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt 2 Department of Anatomy and Embryology, Faculty of Veterinary Medicine, Minia University, Minia, Egypt 3 Department of Forensic Medicine and Toxicology, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt * Corresponding author: E-mail: [email protected] http://dx.doi.org/10.17179/excli2018-1478 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/). ABSTRACT Animal hair examination at a criminal scene may provide valuable information in forensic investigations. How- ever, local reference databases for animal hair identification are rare. In the present study, we provide differential histological analysis of hair of some domestic animals in Upper Egypt. For this purpose, guard hair of large rumi- nants (buffalo, camel and cow), small ruminants (sheep and goat), equine (horse and donkey) and canine (dog and cat) were collected and comparative analysis was performed by light microscopy. Based on the hair cuticle scale pattern, type and diameter of the medulla, and the pigmentation, characteristic differential features of each animal species were identified. The cuticle scale pattern was imbricate in all tested animals except in donkey, in which coronal scales were identified. The cuticle scale margin type, shape and the distance in between were characteristic for each animal species. -

Neural Control of Facial Sweat Gland Secretion in Horses
Iowa State University Capstones, Theses and Creative Components Dissertations Fall 2019 Neural control of facial sweat gland secretion in horses Lu Liu Follow this and additional works at: https://lib.dr.iastate.edu/creativecomponents Part of the Comparative and Evolutionary Physiology Commons, and the Systems and Integrative Physiology Commons Recommended Citation Liu, Lu, "Neural control of facial sweat gland secretion in horses" (2019). Creative Components. 455. https://lib.dr.iastate.edu/creativecomponents/455 This Creative Component is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Creative Components by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Neural control of facial sweat gland secretion in horses Lu Liu 2019.11.26 BMS 599 Creative Component Biomedical Sciences Department Iowa State University Abstract: For sweat glands, it has been shown that sympathetic neurons express a cholinergic/noradrenergic co-phenotype in their innervation in the trunk of mice.(Schütz et al. 2008). It is unknown if facial sweat glands are innervated the same way. Gustatory sweating is an abnormal sweating condition. People can sweat profusely when eating or drinking, or even thinking about eating or drinking. We hypothesize that the facial sweat glands are controlled differently and can be related to parasympathetic neurons from cranial nerves. Some samples from human donors have been tested, but few sweat glands can be targeted since the donors were older people. Thus, it was decided to use horse tissue for this project to obtain greater numbers of sweat glands on the face. -

The Integumentary System
CHAPTER 5: THE INTEGUMENTARY SYSTEM Copyright © 2010 Pearson Education, Inc. OVERALL SKIN STRUCTURE 3 LAYERS Copyright © 2010 Pearson Education, Inc. Figure 5.1 Skin structure. Hair shaft Dermal papillae Epidermis Subpapillary vascular plexus Papillary layer Pore Appendages of skin Dermis Reticular • Eccrine sweat layer gland • Arrector pili muscle Hypodermis • Sebaceous (oil) gland (superficial fascia) • Hair follicle Nervous structures • Hair root • Sensory nerve fiber Cutaneous vascular • Pacinian corpuscle plexus • Hair follicle receptor Adipose tissue (root hair plexus) Copyright © 2010 Pearson Education, Inc. EPIDERMIS 4 (or 5) LAYERS Copyright © 2010 Pearson Education, Inc. Figure 5.2 The main structural features of the skin epidermis. Keratinocytes Stratum corneum Stratum granulosum Epidermal Stratum spinosum dendritic cell Tactile (Merkel) Stratum basale Dermis cell Sensory nerve ending (a) Dermis Desmosomes Melanocyte (b) Melanin granule Copyright © 2010 Pearson Education, Inc. DERMIS 2 LAYERS Copyright © 2010 Pearson Education, Inc. Figure 5.3 The two regions of the dermis. Dermis (b) Papillary layer of dermis, SEM (22,700x) (a) Light micrograph of thick skin identifying the extent of the dermis, (50x) (c) Reticular layer of dermis, SEM (38,500x) Copyright © 2010 Pearson Education, Inc. Figure 5.3a The two regions of the dermis. Dermis (a) Light micrograph of thick skin identifying the extent of the dermis, (50x) Copyright © 2010 Pearson Education, Inc. Q1: The type of gland which secretes its products onto a surface is an _______ gland. 1) Endocrine 2) Exocrine 3) Merocrine 4) Holocrine Copyright © 2010 Pearson Education, Inc. Q2: The embryonic tissue which gives rise to muscle and most connective tissue is… 1) Ectoderm 2) Endoderm 3) Mesoderm Copyright © 2010 Pearson Education, Inc. -

A New Look Into an Old Problem: Keratins As Tools to Investigate Determmanon, Morphogenesis, and Differentiation in Skin
Downloaded from genesdev.cshlp.org on October 10, 2021 - Published by Cold Spring Harbor Laboratory Press A new look into an old problem: keratins as tools to investigate determmanon, morphogenesis, and differentiation in skin Raphael Kopan and Elaine Fuchs Departments of Molecular Genetics and Cell Biology and Biochemistry and Molecular Biology, The University of Chicago, Chicago, Illinois 60637 USA We have investigated keratin and keratin mRNA expression during (1) differentiation of stem cells into epidermis and hair follicles and (2) morphogenesis of follicles. Our results indicate that a type I keratin K14 is expressed early in embryonal basal cells. Subsequently, its expression is elevated in the basal layer of developing epidermis but suppressed in developing matrix cells. This difference represents an early and major biochemical distinction between the two diverging cell types. Moreover, because expression of this keratin is not readily influenced by extracellular regulators or cell culture, it suggests a well-defined and narrow window of development during which an irreversible divergence in basal and matrix cells may take place. In contrast to KI4, which is expressed very early in development and coincident with basal epidermal differentiation, a hair- specific type I keratin and its mRNA is expressed late in hair matrix development and well after follicle morphogenesis. Besides providing an additional developmental difference between epidermal and hair matrix cells, the hair-specific keratins provide the first demonstration that keratin expression may be a consequence rather than a cause of cell organization and differentiation. [Key Words: Hair-specific keratins; keratin mRNA expression; hair follicle morphogenesis] Received September 28, 1988; revised version accepted November 22, 1988. -

The Mammary Bud As a Skin Appendage: Unique and Shared Aspects of Development
J Mammary Gland Biol Neoplasia (2006) 11:187–203 DOI 10.1007/s10911-006-9029-x The Mammary Bud as a Skin Appendage: Unique and Shared Aspects of Development Marja L. Mikkola & Sarah E. Millar Published online: 17 November 2006 # Springer Science + Business Media, Inc. 2006 Abstract Like other skin appendages, the embryonic that can begin to explain the diversity of appendage mammary gland develops via extensive epithelial–mesen- formation, and discuss human genetic diseases that affect chymal interactions. Early stages in embryonic mammary appendage morphogenesis. development strikingly resemble analogous steps in the development of hair follicles and teeth. In each case the Keywords Mammary placode . Mammary bud . first morphological sign of development is a localized Appendage . Hair follicle . Tooth . Ectodermal . thickening in the surface epithelium that subsequently Epidermis . Embryo invaginates to form a mammary, hair follicle or tooth bud. Similar sets of intersecting signaling pathways are involved Abbreviations in patterning the mammary, hair follicle and dental ADULT acro-dermato-ungual-lacrimal-tooth syndrome epithelium, directing placode formation, and controlling APC adenomatous Polyposis Coli bud invagination. Despite these similarities, subsequent AREG Amphiregulin events in the formation of these appendages are diverse. AEC ankyloblepharon-ectodermal dysplasia-clefting The mammary bud extends to form a sprout that begins to syndrome branch upon contact with the mammary fat pad. Hair BCC basal cell carcinoma follicles also extend into the underlying mesenchyme, but BMP bone morphogenetic protein instead of branching, hair follicle epithelium folds around a DKK1 Dickkopf 1 condensation of dermal cells. In contrast, teeth undergo a EDA Ectodysplasin more complex folding morphogenesis. -

Primary Mouse Keratinocyte Cultures Contain Hair Follicle Progenitor Cells with Multiple Differentiation Potential
Primary Mouse Keratinocyte Cultures Contain Hair Follicle Progenitor Cells with Multiple Differentiation Potential Jun Kamimura,*i" David Lee,* Howard P. Baden,* Janice Brissette,* and G. Paolo Dotto* *Cuwneous 13io logy ltese>1 rch Center, Massac husetts General Hospital and Harva rd Medica l School, C harl estown, M arylond, U .S. A.: and ·j·S hiseido R.esca rch Centre, Yokohama, Japan The interfollicular epidermis contains a single type of reassociation of cells. Instead, the reconstituted inter tenninally differentiated keratinocytes, whereas hair follicular epidennis contained distinct colum.nar units, follicles are composed of a minimum of six or seven comprising all the overlying layers and most likely derived distinct types. Whether or not these various populations from a single progenitor cell. In contrast, hair follicles of terminally differentiated keratinocytes originate from were found to be composed of cells of multiple origin, one or more progenitor cells has not been established. A with each population showing a striking localization related and in1portant question is whether keratinocyte to a separate concentric region. The vast majority of reconstituted progenitor cells with a pluripotent potential, able to follicles appeared to derive from a n1initnutn of two or, in a significant fraction of cases, form. not only epidern1is but also hair follicles, can be three progenitor cells, one for the generation of the shaft maintained i11 11itro for any period of time. We have (cuticle, cortex, and n1edulla), one for the inner root addressed these questions using skin reconstitution assays sheath, and the third for the outer root sheath. The with adrnixed populations of genetically labeled, cultured general implications of these findings for epidermis and keratinocytes. -

Intro/Anatomy of Skin, Hair and Nails
- Stratum spinosum 1 – Intro/Anatomy of o Superficial to stratum basale, named for spiny- appearing desmosomes between cells o Keratins 1 and 10 are expressed in this layer and are skin, hair and nails mutated in epidermolytic hyperkeratosis (aka bullous congenital ichthyosiform erythroderma) Vital Functions - Stratum basale o Located just above the basement membrane, is - Sensation, barrier, immune surveillance, UV protection, composed of 10% stem cells thermoregulation o Keratins 5 and 14 are expressed in the basal layer Fun facts and are mutated in patients with epidermolysis bullosa simplex (EBS) - The skin is the largest human organ, 15% of a person’s body weight Major CELL TYPES of the epidermis - Skin cancer = most common cancer worldwide; affects 1 in 5 1. Keratinocytes people (KC) (“squamous cells”, “epidermal cells”) o Make up most of epidermis, produce keratin - Our skin is constantly being renewed, with the epidermis 2. Melanocytes (MC) turning over q40-56 days, results in average person shedding o Neural-crest derived 9 lbs of skin yearly o Normally present in ratio of 1 MC : 10 KC’s Skin thickness varies based on…. o Synthesize and secrete pigment granules called melanosomes - Location: epidermis is thickest on palms/soles at ~ 1.5mm o *** Different races and skin types actually have the (thickness of a penny), thinnest on eyelid/postauricular at ~ same amount of melanoCYTES but differ in the 0.05mm (paper) number, size, type, and distribution of - Age: Skin is relatively thin in children, thickens up until our melanoSOMES, with fairer skin types having more 30’s or 40’s, and then thins out thereafter. -

The Biology and Science of Hair, Skin and Nails Hair There Are
900 E. Hill Ave Suite 380 Knoxville, TN 37915 www.athomeprep.com 1-800-952-0910 The Biology and Science of Hair, Skin and Nails Hair There are approximately 5 million hair follicles on the body with 100-150 thousand located on the scalp. By 22 weeks gestation, the fetus has all of the hair follicles that it will ever have and no more are added during its lifetime. The hair follicles are more dense as a young child and become less so as our body grows. The primary functions of hair are protection against UV rays and heat loss. Hair cells are called trichocytes and are among the most rapidly dividing cells in the body - doubling every 23-72 hours. The trichocytes are located in the hair follicle, which in turn is located in the dermis of the skin. Each hair follicle has a follicular papilla which is fed by capillaries (small blood vessels). The papilla surrounds the bulb from which the hair grows. This entire complex is located in the dermis of the skin overlying the cranium (also known as the scalp). The follicle has two layers, an outer and an inner. The outer layer continues up to the sebaceous gland and the inner follows the hair shaft and ends below the opening of the sebaceous gland. Each follicle also contains an arrector pili muscle which attaches to the outer sheath just below the sebaceous gland. This causes hair to stand on end when you are frightened or scared or cold. The sebaceous gland produces sebum which acts as a natural conditioner. -

Hair Analysis ©2005, 2004, 2003, 2001, 1999 by David A
Hair Analysis ©2005, 2004, 2003, 2001, 1999 by David A. Katz. All rights reserved. Hair can be important physical evidence at a crime scene. Hair normally falls from the body over the course of a day. It will stick to a number of materials, especially fabric and clothing. Hair is not easily destroyed, even with exposure to moisture and decomposition of accompanying tissue. There are three types of hair usually seen in animals: Vibrissa. These are the whiskers of many animals. They are normally tactile and sensitive, such as the whiskers on a cat. Bristle. This is the coarse bristle that provides an animal with a protective coat. These guard hairs can readily be identified by their distinctive appearance and morphology between various animal families. Wool. Wool or fur provides insulation from wet and cold. These fine hairs cover the bodies of all mammals. Head and body hair of humans is classified as intermediate hair combining the characteristics of bristle and wool hairs. Four types of hair appear on the bodies of humans: Primordial hairs appear as early as the 3rd month of gestation, growing on the upper lip, the eyebrows, the palms and soles of the fetus. They gradually disappear and are replaced by softer lanugo hair over the entire body. Lanugo hairs are normally shed after the 6th month of gestation. They are fine, soft, unmedullated, and normally unpigmented hairs. The surface of lanugo hair is smooth with almost indiscriminate scales. It is replaced by vellus and terminal hairs. Lanugo hair is often observed on an aborted fetus and can be useful in investigation of possible infanticide. -
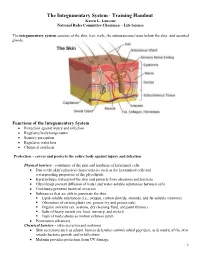
The Integumentary System - Training Handout Karen L
The Integumentary System - Training Handout Karen L. Lancour National Rules Committee Chairman – Life Science The integumentary system consists of the skin, hair, nails, the subcutaneous tissue below the skin, and assorted glands. Functions of the Integumentary System Protection against injury and infection Regulates body temperature Sensory perception Regulates water loss Chemical synthesis Protection – covers and protects the entire body against injury and infection Physical barriers - continuity of the skin and hardness of keratinzed cells Due to the skin’s physical characteristics such as the keratinized cells and waterproofing properties of the glycolipids. Keratin helps waterproof the skin and protects from abrasions and bacteria Glycolipids prevent diffusion of water and water-soluble substances between cells Continuity prevents bacterial invasion Substances that are able to penetrate the skin: . Lipid-soluble substances (i.e., oxygen, carbon dioxide, steroids, and fat-soluble vitamins) . Oleoresins of certain plants (ex. poison ivy and poison oak) . Organic solvents (ex. acetone, dry cleaning fluid, and paint thinner) . Salts of heavy metals (ex. lead, mercury, and nickel) . Topical medications as motion sickness patch Penetration enhancers Chemical barriers - (skin secretion and melanin) Skin secretions such as sebum, human defensins (antimicrobial peptides), acid mantle of the skin retards bacteria growth and/or kills them Melanin provides protection from UV damage 1 Skin secretions (acid mantle) Low pH and sebum slow bacterial growth on skin surface Human defensin – natural antibiotic Cathelicidins – proteins that prevent Strep A infection in wounded skin Melanin – chemical pigment that prevents UV damage Biological Barriers Langerhans’ cells, macrophages, and DNA Langerhans’ cells in epidermis present antigens to lymphocytes Dermal macrophages (2nd line of defense) – attack bacteria and viruses that have penetrated the epidermis Langerhan’s cells and macrophages present in the skin helps activate the body’s immune system. -

The Study of Hair 3
CHAPTER CHAPTER 3 1 2 The Study of Hair 3 4 5 6 NEUTRON ACTIVATION 7 ANALYSIS OF HAIR In 1958, the body of 16-year-old Gaetane 8 Bouchard was discovered in a gravel pit near her home in Edmundston, New Brunswick, 9 across the Canadian–U.S. border from Maine. Numerous stab wounds were found on her body. Witnesses reported seeing Bouchard 10 with her boyfriend John Vollman prior to her disappearance. Circumstantial evidence also 11 linked Vollman with Bouchard. Paint flakes from the place where the couple had been seen together were found in Vollman’s car. 12 Lipstick that matched the color of Bouchard’s lipstick was found on candy in Vollman’s glove 13 compartment. At Bouchard’s autopsy, several strands of hair were found in her hand. This hair was tested 14 using a process known as neutron activation analysis (NAA). NAA tests for the presence and 15 concentration of various elements in a sample. In this case, NAA showed that the hair in Bouchard’s hand contained a ratio of sulfur to 16 phosphorus that was much closer to Vollman’s hair than her own. At the trial, Vollman con- 17 fessed to the murder in light of the hair analysis results. This was the first time NAA hair analysis was used to convict a criminal. ©Stephen J. Krasemann/Photo Researchers, Inc. Investigators search for clues in a gravel pit similar to the one in which Gaetane Bouchard was buried. 48 31559_03_ch03_p048-075.indd 48 10/2/10 2:29:51 Objectives By the end of this chapter you will be able to 3.1 Identify the various parts of a hair. -

Trichohyalin, an Intermediate Filament-Associated Protein of the Hair Follicle Joseph A
Published April 1, 1986 Trichohyalin, an Intermediate Filament-associated Protein of the Hair Follicle Joseph A. Rothnagel and George E. Rogers Department of Biochemistry, University of Adelaide, Adelaide, South Australia 5000, Australia Abstract. A precursor protein associated with the for- human follicles and rat vibrissae inner root sheaths. mation of the citrulline-containing intermediate fila- Tissue immunochemical methods have localized the ments of the hair follicle has been isolated and charac- Mr 190,000 protein to the trichohyalin granules of the terized. The protein, with a molecular weight of developing inner root sheath of the wool follicle. We 190,000, was isolated from sheep wool follicles and propose that the old term trichohyalin be retained to purified until it yielded a single band on a SDS poly- describe this Mr 190,000 protein. Downloaded from acrylamide gel. The Mr 190,000 protein has a high Immunoelectron microscopy has located the Mr content of lysine and glutamic acid/glutamine resi- 190,000 protein to the trichohyalin granules but not dues and is rich in arginine residues, some of which, it to the newly synthesized filaments. This technique has is postulated, undergo a side chain conversion in situ revealed that trichohyalin becomes associated with the into citrulline residues. Polyclonal antibodies were filaments at later stages of development. These results raised to the purified protein, and these cross-react indicate a possible matrix role for trichohyalin. with similar proteins from extracts of guinea pig and jcb.rupress.org HE hardened structures of the medulla and inner root organization, although filamentous structures have been ob- sheath (IRS) ~ tissues of the hair and hair follicle have served (30).