The Mammary Bud As a Skin Appendage: Unique and Shared Aspects of Development
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anatomy and Physiology of Hair
Chapter 2 Provisional chapter Anatomy and Physiology of Hair Anatomy and Physiology of Hair Bilgen Erdoğan ğ AdditionalBilgen Erdo informationan is available at the end of the chapter Additional information is available at the end of the chapter http://dx.doi.org/10.5772/67269 Abstract Hair is one of the characteristic features of mammals and has various functions such as protection against external factors; producing sebum, apocrine sweat and pheromones; impact on social and sexual interactions; thermoregulation and being a resource for stem cells. Hair is a derivative of the epidermis and consists of two distinct parts: the follicle and the hair shaft. The follicle is the essential unit for the generation of hair. The hair shaft consists of a cortex and cuticle cells, and a medulla for some types of hairs. Hair follicle has a continuous growth and rest sequence named hair cycle. The duration of growth and rest cycles is coordinated by many endocrine, vascular and neural stimuli and depends not only on localization of the hair but also on various factors, like age and nutritional habits. Distinctive anatomy and physiology of hair follicle are presented in this chapter. Extensive knowledge on anatomical and physiological aspects of hair can contribute to understand and heal different hair disorders. Keywords: hair, follicle, anatomy, physiology, shaft 1. Introduction The hair follicle is one of the characteristic features of mammals serves as a unique miniorgan (Figure 1). In humans, hair has various functions such as protection against external factors, sebum, apocrine sweat and pheromones production and thermoregulation. The hair also plays important roles for the individual’s social and sexual interaction [1, 2]. -

Hair Histology As a Tool for Forensic Identification of Some Domestic Animal Species
EXCLI Journal 2018;17:663-670 – ISSN 1611-2156 Received: June 28, 2018, accepted: July 02, 2018, published: July 06, 2018 Original article: HAIR HISTOLOGY AS A TOOL FOR FORENSIC IDENTIFICATION OF SOME DOMESTIC ANIMAL SPECIES Yasser A. Ahmed1, Safwat Ali2, Ahmed Ghallab3* 1 Department of Histology, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt 2 Department of Anatomy and Embryology, Faculty of Veterinary Medicine, Minia University, Minia, Egypt 3 Department of Forensic Medicine and Toxicology, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt * Corresponding author: E-mail: [email protected] http://dx.doi.org/10.17179/excli2018-1478 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/). ABSTRACT Animal hair examination at a criminal scene may provide valuable information in forensic investigations. How- ever, local reference databases for animal hair identification are rare. In the present study, we provide differential histological analysis of hair of some domestic animals in Upper Egypt. For this purpose, guard hair of large rumi- nants (buffalo, camel and cow), small ruminants (sheep and goat), equine (horse and donkey) and canine (dog and cat) were collected and comparative analysis was performed by light microscopy. Based on the hair cuticle scale pattern, type and diameter of the medulla, and the pigmentation, characteristic differential features of each animal species were identified. The cuticle scale pattern was imbricate in all tested animals except in donkey, in which coronal scales were identified. The cuticle scale margin type, shape and the distance in between were characteristic for each animal species. -

Smooth Muscle A-Actin Is a Marker for Hair Follicle Dermis in Vivoand in Vitro
Smooth muscle a-actin is a marker for hair follicle dermis in vivo and in vitro COLIN A. B. JAHODA1*, AMANDA J. REYNOLDS2•*, CHRISTINE CHAPONNIER2, JAMES C. FORESTER3 and GIULIO GABBIANI* 1Department of Biological Sciences, University of Dundee, Dundee DD1 4HN, Scotland ^Department of Pathology, University of Geneva, 1211 Geneva 4, Switzerland ^Department of Surgery, Ninewells Hospital and Medical School, Dundee, Scotland * Present address: Department of Biological Sciences, University of Durham, Durham DH1 3LE, England Summary We have examined the expression of smooth muscle cells contained significant quantities of the a-actin a-actin in hair follicles in situ, and in hair follicle isoform. dermal cells in culture by means of immunohisto- The rapid switching on of smooth muscle a-actin chemistry. Smooth muscle a-actin was present in the expression by dermal papilla cells in early culture, dermal sheath component of rat vibrissa, rat pelage contrasts with the behaviour of smooth muscle cells and human follicles. Dermal papilla cells within all in vitro, and has implications for control of ex- types of follicles did not express the antigen. How- pression of the antigen in normal adult systems. The ever, in culture a large percentage of both hair very high percentage of positively marked cultured dermal papilla and dermal sheath cells were stained papilla and sheath cells also provides a novel marker by this antibody. The same cells were negative when of cells from follicle dermis, and reinforces the idea tested with an antibody to desmin. Overall, explant- that they represent a specialized cell population, derived skin fibroblasts had relatively low numbers contributing to the heterogeneity of fibroblast cell of positively marked cells, but those from skin types in the skin dermis, and possibly acting as a regions of high hair-follicle density displayed more source of myofibroblasts during wound healing. -

Neural Control of Facial Sweat Gland Secretion in Horses
Iowa State University Capstones, Theses and Creative Components Dissertations Fall 2019 Neural control of facial sweat gland secretion in horses Lu Liu Follow this and additional works at: https://lib.dr.iastate.edu/creativecomponents Part of the Comparative and Evolutionary Physiology Commons, and the Systems and Integrative Physiology Commons Recommended Citation Liu, Lu, "Neural control of facial sweat gland secretion in horses" (2019). Creative Components. 455. https://lib.dr.iastate.edu/creativecomponents/455 This Creative Component is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Creative Components by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Neural control of facial sweat gland secretion in horses Lu Liu 2019.11.26 BMS 599 Creative Component Biomedical Sciences Department Iowa State University Abstract: For sweat glands, it has been shown that sympathetic neurons express a cholinergic/noradrenergic co-phenotype in their innervation in the trunk of mice.(Schütz et al. 2008). It is unknown if facial sweat glands are innervated the same way. Gustatory sweating is an abnormal sweating condition. People can sweat profusely when eating or drinking, or even thinking about eating or drinking. We hypothesize that the facial sweat glands are controlled differently and can be related to parasympathetic neurons from cranial nerves. Some samples from human donors have been tested, but few sweat glands can be targeted since the donors were older people. Thus, it was decided to use horse tissue for this project to obtain greater numbers of sweat glands on the face. -

Cell Delamination in the Mesencephalic Neural Fold and Its
© 2013. Published by The Company of Biologists Ltd | Development (2013) 140, 4890-4902 doi:10.1242/dev.094680 RESEARCH ARTICLE Cell delamination in the mesencephalic neural fold and its implication for the origin of ectomesenchyme Raymond Teck Ho Lee1, Hiroki Nagai2, Yukiko Nakaya2, Guojun Sheng2, Paul A. Trainor3,4, James A. Weston5 and Jean Paul Thiery1,6,7,* ABSTRACT dorsal neural fold epithelia (Hörstadius, 1950) suggested that the The neural crest is a transient structure unique to vertebrate embryos neural crest is the source of multipotent stem cells that give rise to that gives rise to multiple lineages along the rostrocaudal axis. In a remarkable diversity of cell types, including pigment cells, neurons cranial regions, neural crest cells are thought to differentiate into and glia of the peripheral and enteric nervous systems. In addition, chondrocytes, osteocytes, pericytes and stromal cells, which are the neural crest was widely considered to be the source of collectively termed ectomesenchyme derivatives, as well as pigment mesenchymal connective tissues that entered the branchial arches to and neuronal derivatives. There is still no consensus as to whether form components of the craniofacial skeleton and connective tissue the neural crest can be classified as a homogenous multipotent of the dorsal fin at the trunk axial levels of fishes and amphibia population of cells. This unresolved controversy has important (Hörstadius, 1950; Le Douarin and Kalcheim, 1999; Raven, 1936). implications for the formation of ectomesenchyme and for Grafting studies in avian embryos (Le Douarin and Teillet, 1974; confirmation of whether the neural fold is compartmentalized into Nakamura and Ayer-le Lievre, 1982) suggested that developmental distinct domains, each with a different repertoire of derivatives. -
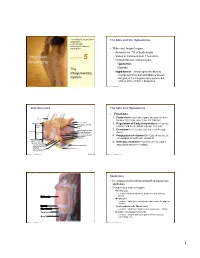
The Integumentary System, but Shares Some of Skin’S Properties
PowerPoint® Lecture Slides The Skin and the Hypodermis prepared by Leslie Hendon University of Alabama, Birmingham • Skin—our largest organ • Accounts for 7% of body weight • Varies in thickness from 1.5–4.4mm C H A P T E R 5 • Divided into two distinct layers • Epidermis The • Dermis Integumentary • Hypodermis—lies deep to the dermis • Composed of areolar and adipose tissues System • Not part of the integumentary system, but shares some of skin’s properties Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. Skin Structure The Skin and Hypodermis • Functions 1. Protection—cushions organs and protects from Hair shaft bumps, chemicals, water loss, UV radiation Dermal papillae Epidermis Subpapillary vascular 2. Regulation of body temperature---Capillary Papillary plexus network and sweat glands regulate heat loss layer Pore Appendages of skin 3. Excretion—urea, salts, and water lost through Dermis Reticular Eccrine sweat gland sweat layer Arrector pili muscle Sebaceous (oil) gland Hair follicle 4. Production of vitamin D---Epidermal cells use Hair root UV radiation to synthesize vitamin D Hypodermis (superficial fascia) 5. Sensory reception—Contains sense organs Nervous structures Sensory nerve fiber Dermal vascular plexus associated with nerve endings Lamellar (Pacinian) corpuscle Adipose tissue Hair follicle receptor (root hair plexus) Copyright © 2011 Pearson Education, Inc. Figure 5.1 Copyright © 2011 Pearson Education, Inc. Figure 5.2 Gross structure of skin and underlying tissues. Epidermis • Is composed of keratinized stratified squamous epithelium • Contains four main cell types • Keratinocytes • Location—stratum spinosum; produce keratin a fibrous protein Epidermis • Melanocytes • Location—basal layer; manufacture and secrete the pigment melanin Dermis • Tactile epithelial cells (Merkel cells) Hypodermis • Location—basal layer; attached to sensory nerve endings Deep fascia • Dendritic cells (Langerhans cells) • Location—stratum spinosum; part of immune system; Muscle macrophage-like Copyright © 2011 Pearson Education, Inc. -

The Integumentary System
CHAPTER 5: THE INTEGUMENTARY SYSTEM Copyright © 2010 Pearson Education, Inc. OVERALL SKIN STRUCTURE 3 LAYERS Copyright © 2010 Pearson Education, Inc. Figure 5.1 Skin structure. Hair shaft Dermal papillae Epidermis Subpapillary vascular plexus Papillary layer Pore Appendages of skin Dermis Reticular • Eccrine sweat layer gland • Arrector pili muscle Hypodermis • Sebaceous (oil) gland (superficial fascia) • Hair follicle Nervous structures • Hair root • Sensory nerve fiber Cutaneous vascular • Pacinian corpuscle plexus • Hair follicle receptor Adipose tissue (root hair plexus) Copyright © 2010 Pearson Education, Inc. EPIDERMIS 4 (or 5) LAYERS Copyright © 2010 Pearson Education, Inc. Figure 5.2 The main structural features of the skin epidermis. Keratinocytes Stratum corneum Stratum granulosum Epidermal Stratum spinosum dendritic cell Tactile (Merkel) Stratum basale Dermis cell Sensory nerve ending (a) Dermis Desmosomes Melanocyte (b) Melanin granule Copyright © 2010 Pearson Education, Inc. DERMIS 2 LAYERS Copyright © 2010 Pearson Education, Inc. Figure 5.3 The two regions of the dermis. Dermis (b) Papillary layer of dermis, SEM (22,700x) (a) Light micrograph of thick skin identifying the extent of the dermis, (50x) (c) Reticular layer of dermis, SEM (38,500x) Copyright © 2010 Pearson Education, Inc. Figure 5.3a The two regions of the dermis. Dermis (a) Light micrograph of thick skin identifying the extent of the dermis, (50x) Copyright © 2010 Pearson Education, Inc. Q1: The type of gland which secretes its products onto a surface is an _______ gland. 1) Endocrine 2) Exocrine 3) Merocrine 4) Holocrine Copyright © 2010 Pearson Education, Inc. Q2: The embryonic tissue which gives rise to muscle and most connective tissue is… 1) Ectoderm 2) Endoderm 3) Mesoderm Copyright © 2010 Pearson Education, Inc. -

Culturing of Melanocytes from the Equine Hair Follicle Outer Root Sheath
processes Article Culturing of Melanocytes from the Equine Hair Follicle Outer Root Sheath Hanluo Li 1,† , Jule Kristin Michler 2,† , Alexander Bartella 1 , Anna Katharina Sander 1, Sebastian Gaus 1, Sebastian Hahnel 3, Rüdiger Zimmerer 1, Jan-Christoph Simon 4, Vuk Savkovic 1,*,‡ and Bernd Lethaus 1,‡ 1 Department of Cranial Maxillofacial Plastic Surgery, University Hospital Leipzig, 04103 Leipzig, Germany; [email protected] (H.L.); [email protected] (A.B.); [email protected] (A.K.S.); [email protected] (S.G.); [email protected] (R.Z.); [email protected] (B.L.) 2 Institute of Veterinary Anatomy, University of Leipzig, 04103 Leipzig, Germany; [email protected] 3 Polyclinic for Dental Prosthetics and Material Sciences, University Hospital Leipzig, 04103 Leipzig, Germany; [email protected] 4 Clinic for Dermatology, Venereology and Allergology, University Hospital Leipzig, 04103 Leipzig, Germany; [email protected] * Correspondence: [email protected]; Tel.: +49-341-97-21115 † The first two authors contributed equally to this work. ‡ These authors contributed equally to this work. Abstract: Hair follicles harbor a heterogeneous regenerative cell pool and represent a putative low- to-non-invasively available source of stem cells. We previously reported a technology for culturing human melanocytes from the hair follicle outer root sheath (ORS) for autologous pigmentation of tissue engineered skin equivalents. This study translated the ORS technology to horses. We de-veloped a culture of equine melanocytes from the ORS (eMORS) from equine forelock hair follicles cultured by means of an analogue human hair follicle-based in vitro methodology. -

(MMP-2) in Ectomesenchyme-Derived Tissues of the Patch Mutant Mouse: Regulation of MMP-2 by PDGF and Effects on Mesenchymal Cell Migration
Developmental Biology 212, 255–263 (1999) Article ID dbio.1999.9373, available online at http://www.idealibrary.com on Diminished Matrix Metalloproteinase 2 (MMP-2) in Ectomesenchyme-Derived Tissues of the Patch Mutant Mouse: Regulation of MMP-2 by PDGF and Effects on Mesenchymal Cell Migration James R. Robbins, Paul G. McGuire, Bernhard Wehrle-Haller,* and Sherry L. Rogers1 Department of Cell Biology and Physiology, University of New Mexico School of Medicine, 149 Basic Medical Sciences Building, Albuquerque, New Mexico 87131; and *De´partment de Pathologie, Centre Medical Universitaire, 1 Rue Michel-Servet, CH-1211 Geneva 4, Switzerland Platelet-derived growth factors (PDGF) regulate cell proliferation, survival, morphology, and migration, as well as deposition and turnover of the extracellular matrix. Important roles for the A form of PDGF (PDGF-A) during connective tissue morphogenesis have been highlighted by the murine Patch mutation, which includes a deletion of the a subunit of the PDGF receptor. Homozygous (Ph/Ph) embryos exhibit multiple connective tissue defects including cleft face (involving the first branchial arch and frontonasal processes), incomplete heart septation, and heart valve abnormalities before they die in utero. Analyses of the cell biology underlying the defects in Ph/Ph embryos have revealed a deficit in a matrix metalloproteinase (MMP-2) and one of its activators (MT-MMP) that are likely to be involved in cell migration and tissue remodeling, two processes necessary for normal cardiac and craniofacial development. Morphogenesis of these structures requires infiltration of ectomesenchymal precursors and their subsequent deposition and remodeling of extracellular matrix components. First branchial arch and heart tissue from E10.5 embryos were examined by gelatin zymography and RT-PCR in order to characterize the expression of MMPs in these tissues. -

The Nail Bed, Part I. the Normal Nail Bed Matrix, Stem Cells, Distal Motion and Anatomy
Central Journal of Dermatology and Clinical Research Review Article *Corresponding author Nardo Zaias, Department of Dermatology Mount Sinai Medical Center, Miami Beach, FL. 33140, 4308 The Nail Bed, Part I. The Normal Alton rd. Suite 750, USA, Email: [email protected] Submitted: 25 November 2013 Nail Bed Matrix, Stem Cells, Distal Accepted: 28 December 2013 Published: 31 December 2013 Copyright Motion and Anatomy © 2014 Zaias Nardo Zaias* OPEN ACCESS Department of Dermatology Mount Sinai Medical Center, USA Abstract The nail bed (NB) has its own matrix that originates from distinctive stem cells. The nail bed matrix stem cells (NBMSC) lie immediately distal to the nail plate (NP) matrix cells and are covered by the keratogenous zone of the most distal NPM (LUNULA). The undivided NBMS cells move distally along the NB basement membrane toward the hyponychium; differentiating and keratinizing at various locations, acting as transit amplifying cells and forming a thin layer of NB corneocytes that contact the overlying onychocytes of the NP, homologous to the inner hair root sheath. At the contact point, the NB corneocytes express CarcinoEmbryonic Antigen (CEA), a glycoprotein-modulating adherence which is also found in hair follicles and tumors. Only when both the NP and the NB are normal do they synchronously move distally. The normal NB keratinizes, expressing keratin K-5 and K-17 without keratohyaline granules. However, during trauma or disease states, it reverts to keratinization with orthokeratosis and expresses K-10, as seen in developmental times. Psoriasis is the only exception. Nail Bed epidermis can express hyperplasia and giant cells in some diseases. -

Keratinocyte-Specific Ablation of the NF-&Kappa
Cell Death and Differentiation (2011) 18, 1845–1853 & 2011 Macmillan Publishers Limited All rights reserved 1350-9047/11 www.nature.com/cdd Keratinocyte-specific ablation of the NF-jB regulatory protein A20 (TNFAIP3) reveals a role in the control of epidermal homeostasis S Lippens1,2, S Lefebvre3, B Gilbert1,2, M Sze1,2, M Devos1,2, K Verhelst1,2, L Vereecke1,2, C Mc Guire1,2, C Gue´rin1,2, P Vandenabeele1,2, M Pasparakis4, ML Mikkola3, R Beyaert1,2,5, W Declercq*,1,2,5 and G van Loo1,2,5 The ubiquitin-editing enzyme A20 (tumor necrosis factor-a-induced protein 3) serves as a critical brake on nuclear factor jB (NF-jB) signaling. In humans, polymorphisms in or near the A20 gene are associated with several inflammatory disorders, including psoriasis. We show here that epidermis-specific A20-knockout mice (A20EKO) develop keratinocyte hyperproliferation, but no signs of skin inflammation, such as immune cell infiltration. However, A20EKO mice clearly developed ectodermal organ abnormalities, including disheveled hair, longer nails and sebocyte hyperplasia. This phenotype resembles that of mice overexpressing ectodysplasin-A1 (EDA-A1) or the ectodysplasin receptor (EDAR), suggesting that A20 negatively controls EDAR signaling. We found that A20 inhibited EDAR-induced NF-jB signaling independent from its de-ubiquitinating activity. In addition, A20 expression was induced by EDA-A1 in embryonic skin explants, in which its expression was confined to the hair placodes, known to be the site of EDAR expression. In summary, our data indicate that EDAR-induced NF-jB levels are controlled by A20, which functions as a negative feedback regulator, to assure proper skin homeostasis and epidermal appendage development. -

Mesodermal Origin of Median Fin Mesenchyme and Tail Muscle In
www.nature.com/scientificreports OPEN Mesodermal origin of median fin mesenchyme and tail muscle in amphibian larvae Received: 29 October 2014 1,2 2 3 2 Accepted: 01 April 2015 Yuka Taniguchi , Thomas Kurth , Daniel Meulemans Medeiros , Akira Tazaki , 2,4 1,2 Published: 18 June 2015 Robert Ramm & Hans-Henning Epperlein Mesenchyme is an embryonic precursor tissue that generates a range of structures in vertebrates including cartilage, bone, muscle, kidney, and the erythropoietic system. Mesenchyme originates from both mesoderm and the neural crest, an ectodermal cell population, via an epithelial to mesenchymal transition (EMT). Because ectodermal and mesodermal mesenchyme can form in close proximity and give rise to similar derivatives, the embryonic origin of many mesenchyme-derived tissues is still unclear. Recent work using genetic lineage tracing methods have upended classical ideas about the contributions of mesodermal mesenchyme and neural crest to particular structures. Using similar strategies in the Mexican axolotl (Ambystoma mexicanum), and the South African clawed toad (Xenopus laevis), we traced the origins of fin mesenchyme and tail muscle in amphibians. Here we present evidence that fin mesenchyme and striated tail muscle in both animals are derived solely from mesoderm and not from neural crest. In the context of recent work in zebrafish, our experiments suggest that trunk neural crest cells in the last common ancestor of tetrapods and ray- finned fish lacked the ability to form ectomesenchyme and its derivatives. According to the germ layer theory of development1 the endoderm gives rise to the digestive tract, the ectoderm generates the nervous system and skin, while muscles and bones are derived from mesoderm.