Reproductive Behavior of Two Argia Spp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Zygoptera: Argia Is Predominantly a Neotropical Genus, the Species
Odonalologica 9 (I): 101 106 March I. 1980 The life cycle of Argia vivida Hagen in the northern part of its range(Zygoptera: Coenagrionidae) G. Pritchard Department of Biology, University of Calgary, Calgary, Alberta, T2N IN4, Canada Received December 3, 1979 A. vivida ranges at least from Mexico to southern Alberta, and larvae live in and cold In both warm streams. warm (geothermally heated) sites in Alberta, Oregon and Idaho, the life cycle is generally univoltine and larval growth is in the larval instar. In sites regulated by a short-day induced diapause penultimate however, the life with naturallyfluctuating temperature regimes, cycle appears to be generallysemivoltine. The role ofdiapause in this 2-year life cycle is presently unknown. INTRODUCTION Argia is predominantly a neotropical genus, the greater number of species being found in South and Central America (WALKER, 1953). The distribution of Argia vivida Hagen ranges at least from Mexico to southern Alberta, and adults have been collected widely in the western United States. Larvae were first described from a cold, spring-fed stream in Washington by KENNEDY (1915), and have subsequently been recorded from sites with naturally fluctuating temperatures (e.g. NIMZ, 1978), as well as from thermal with stable springs higher, more temperatures (PRITCHARD, 1971; PROVONSHA & McCAFFERTY, 1977; NIMZ, 1978). Other members of also occur in thermal & COCKERELL, 1903; the genus springs (NEEDHAM BRUES, 1932; LA RIVERS, 1940; ROBINSON & TURNER. 1975; PRITCHARD, unpublished). In this paper I shall compare life-history data in in for A. vivida cold and warm streams Alberta, Idaho, and Oregon. Year-round data fromthermal pools at Banff, Albertahave been published by PRITCHARD & PELCHAT (1977). -

Happy 75Th Birthday, Nick
ISSN 1061-8503 TheA News Journalrgia of the Dragonfly Society of the Americas Volume 19 12 December 2007 Number 4 Happy 75th Birthday, Nick Published by the Dragonfly Society of the Americas The Dragonfly Society Of The Americas Business address: c/o John Abbott, Section of Integrative Biology, C0930, University of Texas, Austin TX, USA 78712 Executive Council 2007 – 2009 President/Editor in Chief J. Abbott Austin, Texas President Elect B. Mauffray Gainesville, Florida Immediate Past President S. Krotzer Centreville, Alabama Vice President, United States M. May New Brunswick, New Jersey Vice President, Canada C. Jones Lakefield, Ontario Vice President, Latin America R. Novelo G. Jalapa, Veracruz Secretary S. Valley Albany, Oregon Treasurer J. Daigle Tallahassee, Florida Regular Member/Associate Editor J. Johnson Vancouver, Washington Regular Member N. von Ellenrieder Salta, Argentina Regular Member S. Hummel Lake View, Iowa Associate Editor (BAO Editor) K. Tennessen Wautoma, Wisconsin Journals Published By The Society ARGIA, the quarterly news journal of the DSA, is devoted to non-technical papers and news items relating to nearly every aspect of the study of Odonata and the people who are interested in them. The editor especially welcomes reports of studies in progress, news of forthcoming meetings, commentaries on species, habitat conservation, noteworthy occurrences, personal news items, accounts of meetings and collecting trips, and reviews of technical and non-technical publications. Membership in DSA includes a subscription to Argia. Bulletin Of American Odonatology is devoted to studies of Odonata of the New World. This journal considers a wide range of topics for publication, including faunal synopses, behavioral studies, ecological studies, etc. -

The Impacts of Environmental Warming on Odonata: a Review
This is a repository copy of The impacts of environmental warming on Odonata: a review. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/74910/ Article: Hassall, C and Thompson, DJ (2008) The impacts of environmental warming on Odonata: a review. International Journal of Odonatology, 11 (2). 131 - 153 . ISSN 1388-7890 https://doi.org/10.1080/13887890.2008.9748319 Reuse See Attached Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ The effects of environmental warming on Odonata: a review, Hassall and Thompson (2008) - SELF-ARCHIVED COPY This document is the final, reviewed, and revised version of the The effects of environmental warming on Odonata: a review, as submitted to the journal International Journal of Odonatology. It does not include final modifications made during typesetting or copy-editing by the IJO publishing team. This document was archived 12 months after publication of the article in line with the self-archiving policies of the journal International Journal of Odonatology, which can be found here: http://journalauthors.tandf.co.uk/permissions/reusingOwnWork.asp The version of record can be found at the following address: http://www.tandfonline.com/doi/abs/10.1080/13887890.2008.9748319 The paper should be cited as: HASSALL, C. & THOMPSON, D. J. 2008. The impacts of environmental warming on Odonata: a review. International Journal of Odonatology, 11, 131-153. -
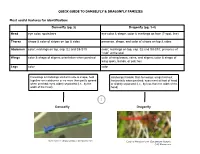
Dragonfly (Pg. 3-4) Head Eye Color
QUICK GUIDE TO DAMSELFLY & DRAGONFLY FAMILIES Most useful features for identification: Damselfly (pg. 2) Dragonfly (pg. 3-4) Head eye color; spots/bars eye color & shape; color & markings on face (T-spot, line) Thorax shape & color of stripes on top & sides presence, shape, and color of stripes on top & sides Abdomen color; markings on top, esp. S2 and S8-S10 color; markings on top, esp. S2 and S8-S10; presence of “club” at the end Wings color & shape of stigma; orientation when perched color of wing bases, veins, and stigma; color & shape of wing spots, bands, or patches Legs color color Forewings & hindwings similar in size & shape, held Hindwings broader than forewings; wings held out together over abdomen or no more than partly spread horizontally when perched; eyes meet at front of head when perched; eyes widely separated (i.e., by the or slightly separated (i.e., by less than the width of the width of the head) head) 1 Damselfly Dragonfly Vivid Dancer (Argia vivida); CAS Mazzacano Cardinal Meadowhawk (Sympetrum illotum); CAS Mazzacano DAMSELFLIES Wings narrow, stalked at base 2 Wings broad, colored, not stalked at base 3 Wings held askew Wings held together Broad-winged Damselfly when perched when perched (Calopterygidae); streams Spreadwing Pond Damsels (Coenagrionidae); (Lestidae); ponds ponds, streams Dancer (Argia); Wings held above abdomen; vivid colors streams 4 Wings held along Bluet (Enallagma); River Jewelwing (Calopteryx aequabilis); abdomen; mostly blue ponds CAS Mazzacano Dark abdomen with blue Forktail (Ischnura); tip; -

Panama, by Nick Donnelly
ISSN 1061-8503 TheA News Journalrgia of the Dragonfly Society of the Americas Volume 23 14 October 2011 Number 3 Published by the Dragonfly Society of the Americas http://www.DragonflySocietyAmericas.org/ ARGIA Vol. 23, No. 3, 14 October 2011 In This Issue .................................................................................................................................................................1 DSA is on Facebook ....................................................................................................................................................1 Calendar of Events ......................................................................................................................................................1 2011 Annual Meeting of DSA held in Fort Collins, Colorado, by Dave Leatherman ...............................................2 Northeast Regional DSA Meeting, by Joshua Rose ...................................................................................................8 2011 Annual Oregon Aeshna Blitz Sets New Records, by Steve Gordon .................................................................10 2012 Annual DSA Meeting: Baldcypress Swamps, Sandy Ponds, Blackwater Rivers, and Clubtails, by Chris Hill ....................................................................................................................................................................12 Northeast Meetings Update, by Bryan Pfeiffer .........................................................................................................12 -

Agrion 17(2) - July 2013 AGRION NEWSLETTER of the WORLDWIDE DRAGONFLY ASSOCIATION
Agrion 17(2) - July 2013 AGRION NEWSLETTER OF THE WORLDWIDE DRAGONFLY ASSOCIATION PATRON: Professor Edward O. Wilson FRS, FRSE Volume 17, Number 2 July 2013 Secretary: Dr. Jessica I. Ware, Assistant Professor, Department of Biological Sciences, 206 Boyden Hall, Rutgers University, 195 University Avenue, Newark, NJ 07102, USA. Email: [email protected]. Editors: Keith D.P. Wilson. 18 Chatsworth Road, Brighton, BN1 5DB, UK. Email: [email protected]. Graham T. Reels. 31 St Anne’s Close, Badger Farm, Winchester, SO22 4LQ, Hants, UK. Email: [email protected]. ISSN 1476-2552 Agrion 17(2) - July 2013 AGRION NEWSLETTER OF THE WORLDWIDE DRAGONFLY ASSOCIATION AGRION is the Worldwide Dragonfly Association’s (WDA’s) newsletter, published twice a year, in January and July. The WDA aims to advance public education and awareness by the promotion of the study and conservation of dragonflies (Odonata) and their natural habitats in all parts of the world. AGRION covers all aspects of WDA’s activities; it communicates facts and knowledge related to the study and conservation of dragonflies and is a forum for news and information exchange for members. AGRION is freely available for downloading from the WDA website at http://ecoevo.uvigo.es/WDA/dragonfly.htm. WDA is a Registered Charity (Not-for-Profit Organization), Charity No. 1066039/0. ________________________________________________________________________________ Editor’s notes Keith Wilson [[email protected]] Conference News The 2013 International Congress of Odonatology was successfully held 17-21, June 2013 in Friesing, Bavaria, German. Pictures and congress information are available at the Congress Website [http://www.ico2013.eu/ crbst_4.html]. A Flickr photo sharing site has been also established [http://www.flickr.com/photos/97838251@ N06/sets/72157634253243888/]. -

To Download the Ranch Dragonfly and Damselfly Checklist
AA ChecklistChecklist ofof DragonfliesDragonflies && DamselfliesDamselflies March 2015 CHICO BASIN RANCH Bill Maynard & Bryan Patrick ragonflies and damselflies together comprise the insect order, Odonata, meaning “toothed Dones”, a reference to their powerful jaws. But, it is their eyes that stand out, in some species almost 30,000 facets comprise a compound eye enabling dragonfly eyes to have the keenest vision in the insect world (80 percent of their brain is used to process visual information). Some dragon- fly species have the largest of all insect eyes. Four ultra flexible wings can rotate on an axis, they can beat together or separately, or, when needed, specialized wings enable dragonflies to hover, fly upside-down, fly backwards, pivot 360 degrees, or fly 100 body lengths per second or about 30 miles per hour. As aquatic larvae they feed voraciously, molting 9 to 17 times before crawling out of the water and hatching into an adult where they live less than two months (only a week for many damselflies). Dragonflies and damselflies, living dinosaurs, evolved during the Carboniferous period, 300 million years ago, where fossil evidence shows they grew to a length of 2.5 feet. Foraging Great White Sharks successfully secure prey 50 percent of the time. African lions are suc- cessful in pursuit of their prey 25 percent of the time, but dragonflies success rate is 95 percent. There are 120 species of Odonata documented in Colorado as of March 15, 2015. The 54 spe- cies identified on Chico Basin Ranch makes it one of the best locations in Colorado to observe and photograph these brightly colored and fascinating insects. -

The Dragonflies (Insecta: Odonata) of the Columbia Basin, British Columbia: Field Surveys, Collections Development and Public Education by Robert A
Living Landscapes The Dragonflies (Insecta: Odonata) of the Columbia Basin, British Columbia: Field Surveys, Collections Development and Public Education by Robert A. Cannings, RBCM, Sydney G. Cannings, CDC, and Leah Ramsay, CDC The Dragonflies (Insecta: Odonata) of the Columbia Basin, British Columbia: Field Surveys, Collections Development and Public Education by: Robert A. Cannings, Royal BC Museum Sydney G. Cannings, B.C. Conservation Data Centre Leah Ramsay, B.C. Conservation Data Centre Table of Contents CIP data Acknowledgements Overview of the Project Introduction to the Dragonflies of the Columbia Basin Dragonfly Habitat in the Columbia Basin Biogeography and Faunal Elements Systematic Review of the Fauna Suborder Zygoptera (Damselflies) Family Calopterygidae (Jewelwings) Family Lestidae (Spreadwings) Family Coenagrionidae (Pond Damsels) Suborder Anisoptera (Dragonflies) Family Aeshnidae (Darners) Family Gomphidae (Clubtails) Family Cordulegastridae (Spiketails) Family Macromiidae (Cruisers) Family Corduliidae (Emeralds) Family Libellulidae (Skimmers) The Effects of Human Activity on Dragonfly Populations Recommendations for Future Inventory, Research and Monitoring References Appendix 1: Checklist of Columbia Basin Dragonflies Appendix 2: Columbia Basin Odonata and Their Faunal Elements Appendix 3: Project Participants Species Distribution Maps and Collecting Data Royal British Columbia Museum 1-888-447-7977 1 675 Belleville Street (250) 356-7226 Copyright 2000 Royal British Columbia Museum Victoria, British Columbia http://www.royalbcmuseum.bc.ca -

The Checklist of Montana Dragonflies & Damselflies
About this Checklist deposit the eggs of further generations. This period River Bluet S c Emma’s Dancer NW,SW,SC o Dragonflies and Damselflies belong to the insect of adult activity is called the Flight Season. Following Enallagma anna M J J A S O N Argia emma M J J A S O N order Odonata, which is split into two suborders: each species is a phenogram [ M J J A S O N ], and Anisoptera – Dragonflies and Zygoptera highlighted in red are the months (May – Nov.) when Familiar Bluet NE,SE c – Damselflies. This checklist includes 53 species of one might expect to see that species during the year. Enallagma civile M J J A S O N Dragonflies (Anisoptera) Dragonflies and 29 species of Damselflies which are Tule Bluet S c known to occur within the state of Montana. Each Species Observed through Oct. 2009 Darners Aeshnidae Enallagma carunculatum M J J A S O N species is listed under its family name and genus. Mosaic Darners Aeshna Common and scientific names are current with those Alkali Bluet S u Damselflies (Zygoptera) Black-tipped Darner NW u set by the Checklist Committee of the Dragonfly Enallagma clausum M J J A S O N Society of the Americas. Aeshna tuberculifera M J J A S O N Broad-winged Damsels Calopterygidae Northern Bluet S c Sedge Darner NW,SW u Jewelwings Calopteryx Enallagma annexum M J J A S O N Distribution Aeshna juncea M J J A S O N To the right of each common name, one or more River Jewelwing NW,SW u Boreal Bluet S c of the following regions will be listed to show the Subarctic Darner NW,SW r Calopteryx aequabilis M J J A S O N Enallagma boreale M J J A S O N approximate distribution of the species within the Aeshna subarctica M J J A S O N Marsh Bluet S c state. -

Odonata: Who They Are and What They Have Done for Us Lately: Classification and Ecosystem Services of Dragonflies
insects Review Odonata: Who They Are and What They Have Done for Us Lately: Classification and Ecosystem Services of Dragonflies Michael L. May Department of Entomology, Rutgers University, New Brunswick, NJ 08901, USA; [email protected] Received: 26 January 2019; Accepted: 22 February 2019; Published: 28 February 2019 Abstract: Odonata (dragonflies and damselflies) are well-known but often poorly understood insects. Their phylogeny and classification have proved difficult to understand but, through use of modern morphological and molecular techniques, is becoming better understood and is discussed here. Although not considered to be of high economic importance, they do provide esthetic/spiritual benefits to humans, and may have some impact as predators of disease vectors and agricultural pests. In addition, their larvae are very important as intermediate or top predators in many aquatic ecosystems. More recently, they have been the objects of study that have yielded new information on the mechanics and control of insect flight. Keywords: damselfly; dragonfly; biomimetic technology; climate warming; ecological indicators; mosquito control; myth and art; phylogeny; predation 1. Introduction Dragonflies are insects that people notice. As adults they are large, diurnal, often colorful, and are swift, acrobatic fliers. They are sufficiently noticeable that they have received numerous folk names, for example, in North America, mosquito hawk, horse stinger, snake doctor, adderbolt, darning needle, among many others [1]. They have become embedded in folklore and mythology in many cultures (see below) and are the subjects of beautiful art and depressingly tacky shlock (whoever started the idea that odonates have curly antennae?!). 2. Classification Despite being so well known as a group, distinctions among species and even higher taxa often seem to be overlooked by the public. -

Zygoptera: Coenagrionidae)
Odonatologica 7 (3) 223-236 September 1, 1978 PROCEEDINGS OF THE FOURTH INTERNATIONAL SYMPOSIUM OF ODONATOLOGY Gainesville, August 1-5, 1977 Part II A mark-recapture study of imaginalEnallagma cyathigerum(Charpentier) andArgia vivida Hagen (Zygoptera: Coenagrionidae) R.W. Garrison 201 Wellman Hall, Division ofEntomology, University ofCalifornia, Berkeley, Ca. 94720, United States Received and Accepted June 27, 1978 A mark-recapture study of the 2 spp. was performed at Del Puerto Cyn., 120 km SSE of San Francisco, Calif., USA, and analyzed by the Jolly and and methods. Movements for of both tended be local. Manly Parr dd spp. to Recapture data for 99 were sparse, probably due to their greater movements. Morisita’s dispersion index for both sexes of both spp. showed the following from least distribution: E. sequence, most to contagious cyathigerum 99, E. cyathigerum dd, A. vivida 99, A. vivida dd. These differences were due to the greater preference of E. cyathigerum for water sites. Female E. cyathige- rum showed a 1:3 ratio of blue to brown-olive morphs, but there appeared to be no assortative mate selection by dd. A significantly higher percentage of 99 was taken in tandem or copula with unmarked dd than marked ones, suggesting that mated 99 entered the water sites already in tandem or copula. Male E. cyathigerum and A. vivida lived an average of 4.68 and 3.02 days, respectively. The Enallagma life span estimate agrees well with reported esti- mates for other Enallagma spp.; but that ofA. vivida is considerably shorter than for estimates for other reported Argia spp. -

Senate Bill No
S.B. 166 SENATE BILL NO. 166–SENATOR WOODHOUSE MARCH 4, 2009 ____________ JOINT SPONSOR: ASSEMBLYMAN STEWART ____________ Referred to Committee on Government Affairs SUMMARY—Designates the official state insect of Nevada. (BDR 19-914) FISCAL NOTE: Effect on Local Government: No. Effect on the State: No. ~ EXPLANATION – Matter in bolded italics is new; matter between brackets [omitted material] is material to be omitted. AN ACT relating to state emblems; designates the Vivid Dancer Damselfly (Argia vivida) as the official state insect of the State of Nevada; and providing other matters properly relating thereto. Legislative Counsel’s Digest: 1 Existing law establishes various official state emblems, including a state bird, 2 state reptile, state animal, state fish and others. (NRS 235.020-235.130) This bill 3 establishes the Vivid Dancer Damselfly (Argia vivida) as the official state insect. 4 This insect was selected through a contest that was open to all fourth grade and 5 Gifted and Talented Education (GATE) classrooms in Nevada to select a state 6 insect because Nevada is one of only eight states in the country that does not have a 7 state insect. The contest was open to public, private, parochial and homeschooled 8 pupils. To participate in the contest, the classes were required to submit a one-page, 9 research-based essay supporting the nomination of an insect found in Nevada, 10 including the pupils’ rationale for why that insect would be a good symbol for the 11 State of Nevada. The 74 classrooms that participated in the contest represented 57 12 schools in seven counties.