Zygoptera: Coenagrionidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Damselflies & Dragonflies of the Tees and Humber Industrial Sites
Damselflies & Dragonflies of the Tees and Humber Industrial Sites Introduction Damselflies and dragonflies belong to the same ‘order’ of insects called the Odonata. Although the adults are flying insects, the other stages of their life cycle are spent in water. Adults lay their eggs on aquatic plants or in the mud of still or slow moving freshwater. The larval or ‘nymph’ form is the immature stage which is wingless. Nymphs eat other aquatic insects and small animals such as tadpoles and small fish. They shed their skin as they grow and larger species can take several years to reach the point Damsel or Dragon? where they become full-grown. At this point The following key points will give an they climb out of the water onto surrounding indication of the main differences vegetation and after a short period the adult between dragonflies and damselflies: insect emerges from the old larval skin. The adults are also fearsome predators, catching • Both have two large ‘compound’ eyes at other flying insects on the wing. Damselflies the front of their head. A dragonfly’s eyes eat smaller species, such as greenfly or are so large that they meet in the middle midges, but a dragonfly’s diet includes larger or at the top of the head, but those of a flying insects such as butterflies, moths and damselfly are smaller and always separate. even smaller dragonflies and damselflies! • Both have four wings, but when resting, damselflies fold their wings back This leaflet is a useful field guide, that covers along their bodies or at 45 degrees, the Odonata species which can be seen in whereas dragonflies rest with their the Tees and Humber industrial areas. -

Dragonflies of La Brenne & Vienne
Dragonflies of La Brenne & Vienne Naturetrek Tour Report 13 - 20 June 2018 Dainty White-faced Darter (Leucorrhinia caudalis) male Yellow-spotted Emerald (Somatochlora flavomaculata) male Report and images by Nick Ransdale Naturetrek Mingledown Barn Wolf's Lane Chawton Alton Hampshire GU34 3HJ UK T: +44 (0)1962 733051 E: [email protected] W: www.naturetrek.co.uk Tour Report Dragonflies of La Brenne & Vienne Tour participants: Nick Ransdale (leader) with six Naturetrek clients Summary This two-centre holiday in central-western France gave an excellent insight into not only the dragonflies but also the abundant butterflies, birds and other wildlife of the region. The first two days were spent in the southern Vienne before we moved to the bizarre landscape of the Pinail reserve, and finally to Mezieres where we spent three days in the Brenne - ‘land of a thousand lakes’. This year's tour started on the cool side at 17-18°C, but settled into a pattern that proved to be ideal for finding and photographing odonata. Due to the sharp eyes, flexibility and optimism of group members, the tour was a resounding success, scoring a total of 44 species (tour average 41), equalling the tour record. The emphasis here is always on getting good, diagnostic views for all participants, something we achieved for all but one species. It was a good year for 'sets' of species this year, with both pincertails, four emerald dragonflies and both whiteface species. Added to this were five fritillary butterfly species, both Emperors (Purple and Lesser Purple), and an outstanding two clearwing moths – both Hornet and Firey. -

Index to Contents
Index to Contents Author(s) Title Year Vol Pages Holland, Sonia Dragonfly Survey Reports – 1. Gloucestershire 1983 1 (1) 1-3 Butler, Stephen Notes on finding larvae of Somatochlora arctica (Zetterstedt) in N. W. Scotland 1983 1 (1) 4-5 Winsland, David Some observations on Erythromma najas (Hansemann) 1983 1 (1) 6 Merritt, R. Is Sympetrum nigrescens Lucas a good species? 1983 1 (1) 7-8 Vick, G. S. Is Sympetrum nigrescens Lucas a good species? 1983 1 (1) 7-8 Merritt, R. Coenagrion mercuriale (Charpentier) with notes on habitat 1983 1 (1) 9-12 Chelmick, D. G. Observations on the ecology and distribution of Oxygastra curtisii (Dale) 1983 1 (2) 11-14 Khan, R. J. Observations of Wood-mice (Apodemus sylvaticus) and Hobby (Falco subbuteo) feeding on dragonflies 1983 1 (2) 15 Marren, P. R. Scarce Species Status Report 2. A review of Coenagrion hastulatum (Charpentier) in Britain 1983 1 (2) 16-19 Merritt, R. Is Sympetrum nigrescens Lucas a good species? 1983 1 (2) 16-19 Mayo, M. C. A. Coenagrion mercuriale (Charpentier) on the flood plains of the River Itchen and River Test in Hampshire 1983 1 (2) 20-21 Welstead, A. R. Coenagrion mercuriale (Charpentier) on the flood plains of the River Itchen and river Test in Hampshire 1983 1 (2) 20-21 Kemp, R. G. Notes and observations on Gomphus vulgatissimus (Linnaeus) on the river Severn and River Thames 1983 1 (2) 22-25 Vick, G. S. Notes and observations on Gomphus vulgatissimus (Linnaeus) on the river Severn and River Thames 1983 1 (2) 22-25 Corbet, P. -

(Zygoptera: Argia Is Predominantly a Neotropical Genus, the Species
Odonalologica 9 (I): 101 106 March I. 1980 The life cycle of Argia vivida Hagen in the northern part of its range(Zygoptera: Coenagrionidae) G. Pritchard Department of Biology, University of Calgary, Calgary, Alberta, T2N IN4, Canada Received December 3, 1979 A. vivida ranges at least from Mexico to southern Alberta, and larvae live in and cold In both warm streams. warm (geothermally heated) sites in Alberta, Oregon and Idaho, the life cycle is generally univoltine and larval growth is in the larval instar. In sites regulated by a short-day induced diapause penultimate however, the life with naturallyfluctuating temperature regimes, cycle appears to be generallysemivoltine. The role ofdiapause in this 2-year life cycle is presently unknown. INTRODUCTION Argia is predominantly a neotropical genus, the greater number of species being found in South and Central America (WALKER, 1953). The distribution of Argia vivida Hagen ranges at least from Mexico to southern Alberta, and adults have been collected widely in the western United States. Larvae were first described from a cold, spring-fed stream in Washington by KENNEDY (1915), and have subsequently been recorded from sites with naturally fluctuating temperatures (e.g. NIMZ, 1978), as well as from thermal with stable springs higher, more temperatures (PRITCHARD, 1971; PROVONSHA & McCAFFERTY, 1977; NIMZ, 1978). Other members of also occur in thermal & COCKERELL, 1903; the genus springs (NEEDHAM BRUES, 1932; LA RIVERS, 1940; ROBINSON & TURNER. 1975; PRITCHARD, unpublished). In this paper I shall compare life-history data in in for A. vivida cold and warm streams Alberta, Idaho, and Oregon. Year-round data fromthermal pools at Banff, Albertahave been published by PRITCHARD & PELCHAT (1977). -

IDF-Report 158 (2021)
IDF International Dragonfly Fund Report Journal of the International Dragonfly Fund 1-22 Xin Yu A survey of Odonata diversity in Zoige wetland, Sichuan Province, China published: 19.03.2021 158 ISSN 1435-3393 The International Dragonfly Fund (IDF) is a scientific society founded in 1996 for the impro vement of odonatological knowledge and the protection of species. Internet: http://www.dragonflyfund.org/ This series intends to publish studies promoted by IDF and to facilitate costefficient and ra pid dissemination of odonatological data. Editorial Work: Holger Hunger, Martin Schorr, Milen Marinov, Rory A. Dow, Oleg E. Kosterin, Vladimir Onishko Layout: Martin Schorr IDF-home page: Holger Hunger Printing: Colour Connection GmbH, Frankfurt Impressum: Publisher: International Dragonfly Fund e.V., Schulstr. 7B, 54314 Zerf, Germany. Email: [email protected] Responsible editor: Martin Schorr Cover picture: Coenagrion lunulatum, male Photographer: Xin Yu Published 19.03.2021 A survey of Odonata diversity in Zoige wetland, Sichuan Province, China Xin Yu College of Life Sciences, Chongqing Normal University, Chongqing, PR China Email: [email protected] Abstract At Zoige alpine Wetland, a total of 10 species belonging to 4 families and 6 genera were recorded. Obvious melanism in Coenagrion lunulatum and feigning death behaviour in Enallagma cyathigerum were observed. A preliminary trial on avoiding behaviour of E. cyathigerum confirmed that feigning death is one of the major strategies to protect them- selves. All these new findings are -

Simultaneous Quaternary Radiations of Three Damselfly Clades Across
vol. 165, no. 4 the american naturalist april 2005 E-Article Simultaneous Quaternary Radiations of Three Damselfly Clades across the Holarctic Julie Turgeon,1,2,* Robby Stoks,1,3,† Ryan A. Thum,1,4,‡ Jonathan M. Brown,5,§ and Mark A. McPeek1,k 1. Department of Biological Sciences, Dartmouth College, the evolution of mate choice in generating reproductive isolation as Hanover, New Hampshire 03755; species recolonized the landscape following deglaciation. These anal- 2. De´partement de Biologie, Universite´ Laval, Que´bec, Que´bec yses suggest that recent climate fluctuations resulted in radiations G1K 7P4, Canada; driven by similar combinations of speciation processes acting in dif- 3. Laboratory of Aquatic Ecology, University of Leuven, Chemin ferent lineages. de Be´riotstraat 32, B-3000 Leuven, Belgium; 4. Department of Ecology and Systematics, Cornell University, Keywords: Enallagma, speciation, radiation, amplified fragment Ithaca, New York 14850; length polymorphism (AFLP), mtDNA, phylogeny. 5. Department of Biology, Grinnell College, Grinnell, Iowa 50112 Submitted October 22, 2004; Accepted December 27, 2004; The fossil record recounts recurrent cycles of mass ex- Electronically published February 9, 2005 tinction immediately followed by rebounds in biodiversity throughout Earth’s history (Jablonski 1986, 1994; Benton 1987; Raup 1991; Sepkoski 1991). A few of these events profoundly reshaped global biodiversity (e.g., the end- abstract: If climate change during the Quaternary shaped the Permian mass extinction erased up to 96% of the world’s macroevolutionary dynamics of a taxon, we expect to see three fea- species; Raup 1979; Jablonski 1994; but see Raup 1991), tures in its history: elevated speciation or extinction rates should date but most of these have been more limited in their taxo- to this time, more northerly distributed clades should show greater nomic scope (Raup 1991). -

Happy 75Th Birthday, Nick
ISSN 1061-8503 TheA News Journalrgia of the Dragonfly Society of the Americas Volume 19 12 December 2007 Number 4 Happy 75th Birthday, Nick Published by the Dragonfly Society of the Americas The Dragonfly Society Of The Americas Business address: c/o John Abbott, Section of Integrative Biology, C0930, University of Texas, Austin TX, USA 78712 Executive Council 2007 – 2009 President/Editor in Chief J. Abbott Austin, Texas President Elect B. Mauffray Gainesville, Florida Immediate Past President S. Krotzer Centreville, Alabama Vice President, United States M. May New Brunswick, New Jersey Vice President, Canada C. Jones Lakefield, Ontario Vice President, Latin America R. Novelo G. Jalapa, Veracruz Secretary S. Valley Albany, Oregon Treasurer J. Daigle Tallahassee, Florida Regular Member/Associate Editor J. Johnson Vancouver, Washington Regular Member N. von Ellenrieder Salta, Argentina Regular Member S. Hummel Lake View, Iowa Associate Editor (BAO Editor) K. Tennessen Wautoma, Wisconsin Journals Published By The Society ARGIA, the quarterly news journal of the DSA, is devoted to non-technical papers and news items relating to nearly every aspect of the study of Odonata and the people who are interested in them. The editor especially welcomes reports of studies in progress, news of forthcoming meetings, commentaries on species, habitat conservation, noteworthy occurrences, personal news items, accounts of meetings and collecting trips, and reviews of technical and non-technical publications. Membership in DSA includes a subscription to Argia. Bulletin Of American Odonatology is devoted to studies of Odonata of the New World. This journal considers a wide range of topics for publication, including faunal synopses, behavioral studies, ecological studies, etc. -

The Impacts of Environmental Warming on Odonata: a Review
This is a repository copy of The impacts of environmental warming on Odonata: a review. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/74910/ Article: Hassall, C and Thompson, DJ (2008) The impacts of environmental warming on Odonata: a review. International Journal of Odonatology, 11 (2). 131 - 153 . ISSN 1388-7890 https://doi.org/10.1080/13887890.2008.9748319 Reuse See Attached Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ The effects of environmental warming on Odonata: a review, Hassall and Thompson (2008) - SELF-ARCHIVED COPY This document is the final, reviewed, and revised version of the The effects of environmental warming on Odonata: a review, as submitted to the journal International Journal of Odonatology. It does not include final modifications made during typesetting or copy-editing by the IJO publishing team. This document was archived 12 months after publication of the article in line with the self-archiving policies of the journal International Journal of Odonatology, which can be found here: http://journalauthors.tandf.co.uk/permissions/reusingOwnWork.asp The version of record can be found at the following address: http://www.tandfonline.com/doi/abs/10.1080/13887890.2008.9748319 The paper should be cited as: HASSALL, C. & THOMPSON, D. J. 2008. The impacts of environmental warming on Odonata: a review. International Journal of Odonatology, 11, 131-153. -

Reproductive Behavior of Two Argia Spp
International Journal of Odonatology 3 (1 ): 85-94, 2000 © 2000 Backhuys Publishers. 85 REPRODUCTIVE BEHAVIOR OF TWO ARGIA SPP. (ODONATA: COENAGRIONIDAE) AT AN ARIZONA STREAM Jon D. Hoekstra1 & Robert L. Smith Department of Entomology, University of Arizona, Tucson, AZ 85721 1Current address Center for Aquatic Ecology, Illinois Natural History Survey, 607 E. Peabody, Champaign, IL 61820 <e-mail: [email protected] Received 11 January 2000; revised 28 January 2000; accepted 14 February 2000. Key words: Argia, Odonata, dragonfly, reproduction, North America. Abstract Here we provide a first report on the reproductive behavior of Argia sabino Garrison and Argia pima Garrison from observations at Sabino Creek, Arizona. Both species reproduce in autumn (September-October) following late summer rainstorms. Tandem pairs of A. sabino submerge to oviposit on rock substrates. The oviposition substrate is abundant and widespread. Male A. sabino defend mate-encounter territories in the morning at boulder fields or rock outcrops away from the stream. Copulation may last 30 minutes or more. Ovipositing females submerge in tandem with males, typically to depths of 10-30 em, and pairs may remain submerged for over 30 minutes. Male submergence with females can be interpreted as contact mate guarding promoted by sperm competition and/or as a male investment in the female's survival and oviposi tion success. We discuss evidence for both possibilities based on field observations. Whereas ovipositional resources for A. sabino are ubiquitous at Sabino Creek, A. pima uses patchily distributed, discrete ovipositional habitats (wetted rootlets of riparian trees at waterfalls and riffles). Males of A. pima employ a mixture of contact and noncontact mate-attendance strategies. -
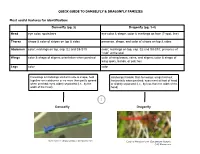
Dragonfly (Pg. 3-4) Head Eye Color
QUICK GUIDE TO DAMSELFLY & DRAGONFLY FAMILIES Most useful features for identification: Damselfly (pg. 2) Dragonfly (pg. 3-4) Head eye color; spots/bars eye color & shape; color & markings on face (T-spot, line) Thorax shape & color of stripes on top & sides presence, shape, and color of stripes on top & sides Abdomen color; markings on top, esp. S2 and S8-S10 color; markings on top, esp. S2 and S8-S10; presence of “club” at the end Wings color & shape of stigma; orientation when perched color of wing bases, veins, and stigma; color & shape of wing spots, bands, or patches Legs color color Forewings & hindwings similar in size & shape, held Hindwings broader than forewings; wings held out together over abdomen or no more than partly spread horizontally when perched; eyes meet at front of head when perched; eyes widely separated (i.e., by the or slightly separated (i.e., by less than the width of the width of the head) head) 1 Damselfly Dragonfly Vivid Dancer (Argia vivida); CAS Mazzacano Cardinal Meadowhawk (Sympetrum illotum); CAS Mazzacano DAMSELFLIES Wings narrow, stalked at base 2 Wings broad, colored, not stalked at base 3 Wings held askew Wings held together Broad-winged Damselfly when perched when perched (Calopterygidae); streams Spreadwing Pond Damsels (Coenagrionidae); (Lestidae); ponds ponds, streams Dancer (Argia); Wings held above abdomen; vivid colors streams 4 Wings held along Bluet (Enallagma); River Jewelwing (Calopteryx aequabilis); abdomen; mostly blue ponds CAS Mazzacano Dark abdomen with blue Forktail (Ischnura); tip; -

Journal Vol 26 No 2, October 2010
J. Br. Dragonfly Society, Volume 26 No. 2, October 2010 Journal of the CONTENTS DAVID CHELMICK - Studying British dragonflies in the British Dragonfly Society 1970s: the wilderness years .............................................. 57 Volume 26 Number 2 October 2010 BARRY NATTRESS - Folding wing behaviour in Cordulagaster boltonii (Donovan) ............................................................. 64 DAVID CHELMICK - Species Review 4: The Scarce Emerald Damselfly Lestes dryas Kirby with notes on the family Lestidae in the Western Palearctic ....................................66 JONATHAN. R. DIXON & DOROTHY E. GENNARD - The influence of meteorological conditions on the flight activity of the Blue-tailed Damselfly Ischnura elegans (Vander Linden), the Azure Damselfly Coenagrion puella (Linnaeus) and the Emerald Damselfly Lestes sponsa (Hansemann) ..... .............................................................................................. 83 ADRIAN J. PARR -. Migrant and dispersive dragonflies in Britain during 2009 ............................................................97 PAM TAYLOR & DAVE SMALLSHIRE - A change in status of the Dainty Damselfly Coenagrion scitulum (Rambur) in the United Kingdom ………....................................................107 Corrigendum ..........................................................................i The aims of the British Dragonfly Society (BDS) are to promote and encourage the study and conservation INSTRUCTIONS TO AUTHORS of Odonata and their natural habitats, especially in the -

Coenagrionids Enallagma Cyathigerum (Charpentier) and Ischnura
Odonatologica 16(4): 375-378 December I, 1987 SHORT COMMUNICATIONS New external morphologicalcharacters for distinguishinglarvae of Enallagma cyathigerum (Charpentier) and Ischnura elegans (Vander Linden) (Zygoptera: Coenagrionidae) S.H. Chowdhury¹ and Philip+S. Corbet² 'Department of Zoology, University ofChittagong, Chittagong, Bangladesh 2 Department of Biological Sciences, University of Dundee, Dundee, DDI 4HN, United Kingdom Received and Accepted March 18, 1987 External morphological characters are described which permit larvae of the 2 spp. instars without to be distinguishedreliably in most, and perhaps all, injuring orkilling abdomen lamellae. specimens. The characters occur on the head, thorax, and caudal INTRODUCTION The small coenagrionids Enallagma cyathigerum (Charpentier) and Ischnura elegans (Vander Linden) are common throughout most of Europe where they frequently occur together in a wide variety ofstanding waters. Unlike I. elegans, which is confinedto the PalaearcticRegion and extends from western Europe to Japan, E. cyathigerum is circumboreal and in the Nearctic Region may coexist with other species of Ischnura (e.g. PILON, 1980). In the in northern Britain (see HAMMOND, many habitats, principally 1983), in which the only other Zygoptera are Lestes sponsa (Hansemann) and Pyrr- which it hosoma nymphula (Sulzer) (both of are easily recognised as larvae) can be especially useful to be able to distinguish larvae of E. cyathigerum and /. elegans. Hitherto the external characters proposed for this purpose have been on the caudal lamella(LUCAS, 1925,* 1930) and the labial palpus (GARDNER, 1954). The characters on the caudal lamella are the relative extent ofspiniform setae on upperand lower margins (GARDNER, 1954, p. 161, couplet 2) and the shape 376 S.H.