(Zygoptera: Argia Is Predominantly a Neotropical Genus, the Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Argia the News Journal of the Dragonfly Society of the Americas
ISSN 1061-8503 TheA News Journalrgia of the Dragonfly Society of the Americas Volume 22 17 December 2010 Number 4 Published by the Dragonfly Society of the Americas http://www.DragonflySocietyAmericas.org/ ARGIA Vol. 22, No. 4, 17 December 2010 In This Issue .................................................................................................................................................................1 Calendar of Events ......................................................................................................................................................1 Minutes of the 2010 Annual Meeting of the Dragonfly Society of the Americas, by Steve Valley ............................2 2010 Treasurer’s Report, by Jerrell J. Daigle ................................................................................................................2 Enallagma novaehispaniae Calvert (Neotropical Bluet), Another New Species for Arizona, by Rich Bailowitz ......3 Photos Needed ............................................................................................................................................................3 Lestes australis (Southern Spreadwing), New for Arizona, by Rich Bailowitz ...........................................................4 Ischnura barberi (Desert Forktail) Found in Oregon, by Jim Johnson ........................................................................4 Recent Discoveries in Montana, by Nathan S. Kohler ...............................................................................................5 -

Cumulative Index of ARGIA and Bulletin of American Odonatology
Cumulative Index of ARGIA and Bulletin of American Odonatology Compiled by Jim Johnson PDF available at http://odonata.bogfoot.net/docs/Argia-BAO_Cumulative_Index.pdf Last updated: 14 February 2021 Below are titles from all issues of ARGIA and Bulletin of American Odonatology (BAO) published to date by the Dragonfly Society of the Americas. The purpose of this listing is to facilitate the searching of authors and title keywords across all issues in both journals, and to make browsing of the titles more convenient. PDFs of ARGIA and BAO can be downloaded from https://www.dragonflysocietyamericas.org/en/publications. The most recent three years of issues for both publications are only available to current members of the Dragonfly Society of the Americas. Contact Jim Johnson at [email protected] if you find any errors. ARGIA 1 (1–4), 1989 Welcome to the Dragonfly Society of America Cook, C. 1 Society's Name Revised Cook, C. 2 DSA Receives Grant from SIO Cook, C. 2 North and Central American Catalogue of Odonata—A Proposal Donnelly, T.W. 3 US Endangered Species—A Request for Information Donnelly, T.W. 4 Odonate Collecting in the Peruvian Amazon Dunkle, S.W. 5 Collecting in Costa Rica Dunkle, S.W. 6 Research in Progress Garrison, R.W. 8 Season Summary Project Cook, C. 9 Membership List 10 Survey of Ohio Odonata Planned Glotzhober, R.C. 11 Book Review: The Dragonflies of Europe Cook, C. 12 Book Review: Dragonflies of the Florida Peninsula, Bermuda and the Bahamas Cook, C. 12 Constitution of the Dragonfly Society of America 13 Exchanges and Notices 15 General Information About the Dragonfly Society of America (DSA) Cook, C. -

Impact of Environmental Changes on the Behavioral Diversity of the Odonata (Insecta) in the Amazon Bethânia O
www.nature.com/scientificreports OPEN Impact of environmental changes on the behavioral diversity of the Odonata (Insecta) in the Amazon Bethânia O. de Resende1,2*, Victor Rennan S. Ferreira1,2, Leandro S. Brasil1, Lenize B. Calvão2,7, Thiago P. Mendes1,6, Fernando G. de Carvalho1,2, Cristian C. Mendoza‑Penagos1, Rafael C. Bastos1,2, Joás S. Brito1,2, José Max B. Oliveira‑Junior2,3, Karina Dias‑Silva2, Ana Luiza‑Andrade1, Rhainer Guillermo4, Adolfo Cordero‑Rivera5 & Leandro Juen1,2 The odonates are insects that have a wide range of reproductive, ritualized territorial, and aggressive behaviors. Changes in behavior are the frst response of most odonate species to environmental alterations. In this context, the primary objective of the present study was to assess the efects of environmental alterations resulting from shifts in land use on diferent aspects of the behavioral diversity of adult odonates. Fieldwork was conducted at 92 low‑order streams in two diferent regions of the Brazilian Amazon. To address our main objective, we measured 29 abiotic variables at each stream, together with fve morphological and fve behavioral traits of the resident odonates. The results indicate a loss of behaviors at sites impacted by anthropogenic changes, as well as variation in some morphological/behavioral traits under specifc environmental conditions. We highlight the importance of considering behavioral traits in the development of conservation strategies, given that species with a unique behavioral repertoire may sufer specifc types of extinction pressure. Te enormous variety of behavior exhibited by most animals has inspired human thought, arts, and Science for centuries, from rupestrian paintings to the Greek philosophers. -

Happy 75Th Birthday, Nick
ISSN 1061-8503 TheA News Journalrgia of the Dragonfly Society of the Americas Volume 19 12 December 2007 Number 4 Happy 75th Birthday, Nick Published by the Dragonfly Society of the Americas The Dragonfly Society Of The Americas Business address: c/o John Abbott, Section of Integrative Biology, C0930, University of Texas, Austin TX, USA 78712 Executive Council 2007 – 2009 President/Editor in Chief J. Abbott Austin, Texas President Elect B. Mauffray Gainesville, Florida Immediate Past President S. Krotzer Centreville, Alabama Vice President, United States M. May New Brunswick, New Jersey Vice President, Canada C. Jones Lakefield, Ontario Vice President, Latin America R. Novelo G. Jalapa, Veracruz Secretary S. Valley Albany, Oregon Treasurer J. Daigle Tallahassee, Florida Regular Member/Associate Editor J. Johnson Vancouver, Washington Regular Member N. von Ellenrieder Salta, Argentina Regular Member S. Hummel Lake View, Iowa Associate Editor (BAO Editor) K. Tennessen Wautoma, Wisconsin Journals Published By The Society ARGIA, the quarterly news journal of the DSA, is devoted to non-technical papers and news items relating to nearly every aspect of the study of Odonata and the people who are interested in them. The editor especially welcomes reports of studies in progress, news of forthcoming meetings, commentaries on species, habitat conservation, noteworthy occurrences, personal news items, accounts of meetings and collecting trips, and reviews of technical and non-technical publications. Membership in DSA includes a subscription to Argia. Bulletin Of American Odonatology is devoted to studies of Odonata of the New World. This journal considers a wide range of topics for publication, including faunal synopses, behavioral studies, ecological studies, etc. -

The Impacts of Environmental Warming on Odonata: a Review
This is a repository copy of The impacts of environmental warming on Odonata: a review. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/74910/ Article: Hassall, C and Thompson, DJ (2008) The impacts of environmental warming on Odonata: a review. International Journal of Odonatology, 11 (2). 131 - 153 . ISSN 1388-7890 https://doi.org/10.1080/13887890.2008.9748319 Reuse See Attached Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ The effects of environmental warming on Odonata: a review, Hassall and Thompson (2008) - SELF-ARCHIVED COPY This document is the final, reviewed, and revised version of the The effects of environmental warming on Odonata: a review, as submitted to the journal International Journal of Odonatology. It does not include final modifications made during typesetting or copy-editing by the IJO publishing team. This document was archived 12 months after publication of the article in line with the self-archiving policies of the journal International Journal of Odonatology, which can be found here: http://journalauthors.tandf.co.uk/permissions/reusingOwnWork.asp The version of record can be found at the following address: http://www.tandfonline.com/doi/abs/10.1080/13887890.2008.9748319 The paper should be cited as: HASSALL, C. & THOMPSON, D. J. 2008. The impacts of environmental warming on Odonata: a review. International Journal of Odonatology, 11, 131-153. -

Reproductive Behavior of Two Argia Spp
International Journal of Odonatology 3 (1 ): 85-94, 2000 © 2000 Backhuys Publishers. 85 REPRODUCTIVE BEHAVIOR OF TWO ARGIA SPP. (ODONATA: COENAGRIONIDAE) AT AN ARIZONA STREAM Jon D. Hoekstra1 & Robert L. Smith Department of Entomology, University of Arizona, Tucson, AZ 85721 1Current address Center for Aquatic Ecology, Illinois Natural History Survey, 607 E. Peabody, Champaign, IL 61820 <e-mail: [email protected] Received 11 January 2000; revised 28 January 2000; accepted 14 February 2000. Key words: Argia, Odonata, dragonfly, reproduction, North America. Abstract Here we provide a first report on the reproductive behavior of Argia sabino Garrison and Argia pima Garrison from observations at Sabino Creek, Arizona. Both species reproduce in autumn (September-October) following late summer rainstorms. Tandem pairs of A. sabino submerge to oviposit on rock substrates. The oviposition substrate is abundant and widespread. Male A. sabino defend mate-encounter territories in the morning at boulder fields or rock outcrops away from the stream. Copulation may last 30 minutes or more. Ovipositing females submerge in tandem with males, typically to depths of 10-30 em, and pairs may remain submerged for over 30 minutes. Male submergence with females can be interpreted as contact mate guarding promoted by sperm competition and/or as a male investment in the female's survival and oviposi tion success. We discuss evidence for both possibilities based on field observations. Whereas ovipositional resources for A. sabino are ubiquitous at Sabino Creek, A. pima uses patchily distributed, discrete ovipositional habitats (wetted rootlets of riparian trees at waterfalls and riffles). Males of A. pima employ a mixture of contact and noncontact mate-attendance strategies. -
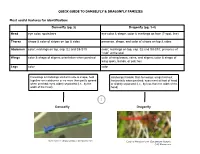
Dragonfly (Pg. 3-4) Head Eye Color
QUICK GUIDE TO DAMSELFLY & DRAGONFLY FAMILIES Most useful features for identification: Damselfly (pg. 2) Dragonfly (pg. 3-4) Head eye color; spots/bars eye color & shape; color & markings on face (T-spot, line) Thorax shape & color of stripes on top & sides presence, shape, and color of stripes on top & sides Abdomen color; markings on top, esp. S2 and S8-S10 color; markings on top, esp. S2 and S8-S10; presence of “club” at the end Wings color & shape of stigma; orientation when perched color of wing bases, veins, and stigma; color & shape of wing spots, bands, or patches Legs color color Forewings & hindwings similar in size & shape, held Hindwings broader than forewings; wings held out together over abdomen or no more than partly spread horizontally when perched; eyes meet at front of head when perched; eyes widely separated (i.e., by the or slightly separated (i.e., by less than the width of the width of the head) head) 1 Damselfly Dragonfly Vivid Dancer (Argia vivida); CAS Mazzacano Cardinal Meadowhawk (Sympetrum illotum); CAS Mazzacano DAMSELFLIES Wings narrow, stalked at base 2 Wings broad, colored, not stalked at base 3 Wings held askew Wings held together Broad-winged Damselfly when perched when perched (Calopterygidae); streams Spreadwing Pond Damsels (Coenagrionidae); (Lestidae); ponds ponds, streams Dancer (Argia); Wings held above abdomen; vivid colors streams 4 Wings held along Bluet (Enallagma); River Jewelwing (Calopteryx aequabilis); abdomen; mostly blue ponds CAS Mazzacano Dark abdomen with blue Forktail (Ischnura); tip; -

Dragonflies and Damselflies Havasu National Wildlife Refuge
U.S. Fish & Wildlife Service Dragonflies and Damselflies Havasu National Wildlife Refuge Dragonfly and damselfly at Havasu National Wildlife Refuge There are twenty-five dragonfly and damselfly species listed at the 37,515 acre Havasu National Wildlife Refuge, one of more than 540 refuges throughout the United States. These National Wildlife Refuges are administered by the Department of the Interior, Fish and Wildlife Service. The Fish and Wildlife Service mission is to work with others “to conserve fish and wildlife and their habitat.” General Information Havasu National Wildlife Refuge encompasses 37,515 acres adjacent to the Colorado River. Topock Marsh, Topock Gorge, and the Havasu Wilderness constitute the three major portions of the refuge. Dragonflies, an important Male blue-ringed dancer sedula indicator of water quality, can be found © Dave Welling Photography on the refuge, primarily in Topock Marsh Libellula luctuosa Tramea onusta and Topock Gorge. Dragonflies can be Widow Skimmer Red Saddlebags viewed on the refuge year-round, with hot, sunny days providing some of the L. pulchella Pond Damsels–Dancers (Coenagrionidae) best viewing. Sixty-three dragonfly and Twelve-spotted Skimmer Argia moesta damsel species have been identified in Powdered Dancer Mohave County, Arizona. Visitors are L. saturate encouraged to contact refuge staff with a Flame Skimmer Argia sedula description or photograph, if an unlisted Blue-ringed Dancer species is observed. Pachydiplax longipennis Blue Dasher Enallagma civile Family Familiar Bluet Scientific -

Panama, by Nick Donnelly
ISSN 1061-8503 TheA News Journalrgia of the Dragonfly Society of the Americas Volume 23 14 October 2011 Number 3 Published by the Dragonfly Society of the Americas http://www.DragonflySocietyAmericas.org/ ARGIA Vol. 23, No. 3, 14 October 2011 In This Issue .................................................................................................................................................................1 DSA is on Facebook ....................................................................................................................................................1 Calendar of Events ......................................................................................................................................................1 2011 Annual Meeting of DSA held in Fort Collins, Colorado, by Dave Leatherman ...............................................2 Northeast Regional DSA Meeting, by Joshua Rose ...................................................................................................8 2011 Annual Oregon Aeshna Blitz Sets New Records, by Steve Gordon .................................................................10 2012 Annual DSA Meeting: Baldcypress Swamps, Sandy Ponds, Blackwater Rivers, and Clubtails, by Chris Hill ....................................................................................................................................................................12 Northeast Meetings Update, by Bryan Pfeiffer .........................................................................................................12 -

Agrion 17(2) - July 2013 AGRION NEWSLETTER of the WORLDWIDE DRAGONFLY ASSOCIATION
Agrion 17(2) - July 2013 AGRION NEWSLETTER OF THE WORLDWIDE DRAGONFLY ASSOCIATION PATRON: Professor Edward O. Wilson FRS, FRSE Volume 17, Number 2 July 2013 Secretary: Dr. Jessica I. Ware, Assistant Professor, Department of Biological Sciences, 206 Boyden Hall, Rutgers University, 195 University Avenue, Newark, NJ 07102, USA. Email: [email protected]. Editors: Keith D.P. Wilson. 18 Chatsworth Road, Brighton, BN1 5DB, UK. Email: [email protected]. Graham T. Reels. 31 St Anne’s Close, Badger Farm, Winchester, SO22 4LQ, Hants, UK. Email: [email protected]. ISSN 1476-2552 Agrion 17(2) - July 2013 AGRION NEWSLETTER OF THE WORLDWIDE DRAGONFLY ASSOCIATION AGRION is the Worldwide Dragonfly Association’s (WDA’s) newsletter, published twice a year, in January and July. The WDA aims to advance public education and awareness by the promotion of the study and conservation of dragonflies (Odonata) and their natural habitats in all parts of the world. AGRION covers all aspects of WDA’s activities; it communicates facts and knowledge related to the study and conservation of dragonflies and is a forum for news and information exchange for members. AGRION is freely available for downloading from the WDA website at http://ecoevo.uvigo.es/WDA/dragonfly.htm. WDA is a Registered Charity (Not-for-Profit Organization), Charity No. 1066039/0. ________________________________________________________________________________ Editor’s notes Keith Wilson [[email protected]] Conference News The 2013 International Congress of Odonatology was successfully held 17-21, June 2013 in Friesing, Bavaria, German. Pictures and congress information are available at the Congress Website [http://www.ico2013.eu/ crbst_4.html]. A Flickr photo sharing site has been also established [http://www.flickr.com/photos/97838251@ N06/sets/72157634253243888/]. -

To Download the Ranch Dragonfly and Damselfly Checklist
AA ChecklistChecklist ofof DragonfliesDragonflies && DamselfliesDamselflies March 2015 CHICO BASIN RANCH Bill Maynard & Bryan Patrick ragonflies and damselflies together comprise the insect order, Odonata, meaning “toothed Dones”, a reference to their powerful jaws. But, it is their eyes that stand out, in some species almost 30,000 facets comprise a compound eye enabling dragonfly eyes to have the keenest vision in the insect world (80 percent of their brain is used to process visual information). Some dragon- fly species have the largest of all insect eyes. Four ultra flexible wings can rotate on an axis, they can beat together or separately, or, when needed, specialized wings enable dragonflies to hover, fly upside-down, fly backwards, pivot 360 degrees, or fly 100 body lengths per second or about 30 miles per hour. As aquatic larvae they feed voraciously, molting 9 to 17 times before crawling out of the water and hatching into an adult where they live less than two months (only a week for many damselflies). Dragonflies and damselflies, living dinosaurs, evolved during the Carboniferous period, 300 million years ago, where fossil evidence shows they grew to a length of 2.5 feet. Foraging Great White Sharks successfully secure prey 50 percent of the time. African lions are suc- cessful in pursuit of their prey 25 percent of the time, but dragonflies success rate is 95 percent. There are 120 species of Odonata documented in Colorado as of March 15, 2015. The 54 spe- cies identified on Chico Basin Ranch makes it one of the best locations in Colorado to observe and photograph these brightly colored and fascinating insects. -

The News Journal of the Dragonfly
ISSN 1061-8503 TheA News Journalrgia of the Dragonfly Society of the Americas Volume 23 1 July 2011 Number 2 Published by the Dragonfly Society of the Americas http://www.DragonflySocietyAmericas.org/ ARGIA Vol. 23, No. 2, 1 July 2011 In This Issue .................................................................................................................................................................1 Calendar of Events ......................................................................................................................................................1 2011 Ohio Odonata Society Meeting .........................................................................................................................2 CalOdes/DSA California Dragonfly Blitz 2011, by Kathy Biggs ..............................................................................3 Dragons and Damsels to Meet in Reno: Upcoming Symposium on Odonata at the Entomological Society of America in Reno, Nevada, 2011, by Seth Bybee and Jessica Ware.....................................................................4 2011 Southeastern Regional DSA Meeting Summary, by Giff Beaton and Marion Dobbs .....................................4 Some Unusual Sightings in the Northeast, by Sue and John Gregoire ......................................................................6 Ischnura perparva (Western Forktail), New to Iowa, by Steve Hummel ....................................................................7 Incredible New Insect Discovered!.............................................................................................................................7