Long- and Short-Term Impact of Temperature on Snake Detection in the Wild: Further Evidence from the Snake Hemorrhois Hippocrepis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NOTES on REPTILES and AMPHIBIANS of NORTHEASTERN GREECEAND the ISLAND of SAMOTHRAKI DAVID BUTTLE 2 Manchester Place, Norwich NR2 2SH, England INTRODUCTION
British Herpetological Society Bulletin, No. 29, 1989. NOTES ON REPTILES AND AMPHIBIANS OF NORTHEASTERN GREECEAND THE ISLAND OF SAMOTHRAKI DAVID BUTTLE 2 Manchester Place, Norwich NR2 2SH, England INTRODUCTION The northeasternGreek mainland was visited for seventeendays, 3lst May to l6th June 1988, during which sixteen reptile and four amphibian species were observed. A further four days, 18th to 21st June, were spent on the northeast Aegean island of Samothraki, resulting in nine reptile and two amphibian species being recorded. On several days, particularly during the latter part of the trip, mid day air temperatures exceeded30'C and consequently searching proved more productive during the cooler hours of early morning and late afternoon. A checklist of the Greek reptiles and amphibians was presented by Ondrias (1968), much of it basedon records in the classicworks of Werner (1938) and Wettstein (1953, 1957).More recently Chondropoulos has been working on an updated checklist of Greek reptiles, the first part of which, on the lizards, was published in 1986.A comparitively small amount of recent research has been carried out in the northeastern Greek mainland and the herpetofauna of the large northeast Aegean islands, including Samothraki, has received little attention. PRINCIPAL SITES The locations of the eight mainland areas investigated and Samothraki island are shown in Figure l. AREA 1 Filippi. Dry rocky hills near ancient ruins. AREA 2 Kavalla. Well vegetated rocky hillsides and agricultural areas to the east of town. AREA 3 Nestos river. Well vegetated river banks. Also rocky scrub covered hills near villages of Toxotes and Galani. AREA 4 Kutson. -

Snake Charming and the Exploitation of Snakes in Morocco
Snake charming and the exploitation of snakes in Morocco J UAN M. PLEGUEZUELOS,MÓNICA F ERICHE,JOSÉ C. BRITO and S OUMÍA F AHD Abstract Traditional activities that potentially threaten bio- also for clothing, tools, medicine and pets, as well as in diversity represent a challenge to conservationists as they try magic and religious activities (review in Alves & Rosa, to reconcile the cultural dimensions of such activities. ). Vertebrates, particularly reptiles, have frequently Quantifying the impact of traditional activities on biodiver- been used for traditional medicine. Alves et al. () iden- sity is always helpful for decision making in conservation. In tified reptile species ( families, genera) currently the case of snake charming in Morocco, the practice was in- used in traditional folk medicine, % of which are included troduced there years ago by the religious order the on the IUCN Red List (IUCN, ) and/or the CITES Aissawas, and is now an attraction in the country’s growing Appendices (CITES, ). Among the reptile species tourism industry. As a consequence wild snake populations being used for medicine, % are snakes. may be threatened by overexploitation. The focal species for Snakes have always both fascinated and repelled people, snake charming, the Egyptian cobra Naja haje, is undergo- and the reported use of snakes in magic and religious activ- ing both range and population declines. We estimated the ities is global (Alves et al., ). The sacred role of snakes level of exploitation of snakes based on field surveys and may be related to a traditional association with health and questionnaires administered to Aissawas during – eternity in some cultures (Angeletti et al., ) and many , and compared our results with those of a study con- species are under pressure from exploitation as a result ducted years previously. -

Khalladi-Bpp Anexes-Arabic.Pdf
Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 107 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 108 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 109 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 110 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 111 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 112 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 113 The IUCN Red List Categories and Criteria are intended to be an easily and widely understood system for classifying species at high risk of global extinction. The IUCN Red List is categorized in the following Categories: • Extinct (EX): A taxon is Extinct when there is no reasonable doubt that the last individual has died. A taxon is presumed Extinct when exhaustive surveys in known and/or expected habitat, at appropriate times (diurnal, seasonal, annual), throughout its historic range have failed to record an individual. Surveys should be over a time frame appropriate to the taxon’s life cycle and life form. Khalladi Windfarm and Power Line Projects 114 Biodiversity Protection Plan, July 2015 • Extinct in the Wild (EW): A taxon is Extinct in the Wild when it is known only to survive in cultivation, in captivity or as a naturalized population (or populations) well outside the past range. A taxon is presumed Extinct in the Wild when exhaustive surveys in known and/or expected habitat, at appropriate times (diurnal, seasonal, annual), throughout its historic range have failed to record an individual. -
B I R D W a T C H I N G •
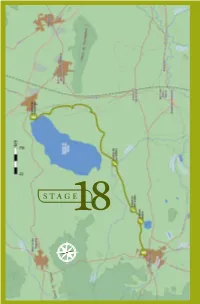
18 . F UENTE DE PIEDRA - CAMPILLOS STAGE18 E S N O 158 BIRDWATCHING • GR-249 Great Path of Malaga F UENTE DE PIEDRA - CAMPILLOS 18 . STAGE 18 Fuente de Piedra - Campillos L O C A T I O N he José Antonio Valverde Visitor´s Centre at the TReserva Natural de la Laguna de Fuente de Piedra is the starting point of Stage 18. Taking the direction south around the taken up mainly by olive trees and grain. eastern side of the salt water lagoon This type of environment will continue to you will be walking through farmland the end of this stage and it determines until the end of this stage in Campillos the species of birds which can be seen village. The 15, 7 km of Stage 18 will here. You will be crossing a stream and allow you to discover this wetland well then walking along the two lakes which known at national level in Spain, and will make your Stage 18 bird list fill cultivated farmland which creates a up with highly desirable species. The steppe-like environment. combination of wetland and steppe creates very valuable habitats with DESCRIPTION a rare composition of taxa unique at European level. ABOUT THE BIRDLIFE: Stage 18 begins at the northern tip HIGHLIGHTED SPECIES of the lagoon where you take direction Neither the length, diffi culty level or south through agricultural environment, elevation gain of this stage is particularly DID YOU KNOW? ecilio Garcia de la Leña, in Conversation 9th of “Historical Conversations of Malaga” published by Cristóbal Medina Conde (1726-1798) and entitled «About Animal Kingdom of Malaga and some Places in its Bishopric», Ccomments: «…In some lagoons, along sea shores and river banks some large and beautiful birds breed, called Flamingos and Phoenicopteros according to the ancients...», after a description of the bird´s anatomy he adds: «The Romans appreciated the bird greatly, especially its tongue which was served as an exquisite dish…». -

A Record of Thanatosis Behaviour in Coronella Girondica (Reptilia: Colubridae) Arancha De Castro-Expósito1, Francisco Guerrero1,2 & Enrique García-Muñoz1,3,4,*
36 Bol. Asoc. Herpetol. Esp. (2017) 28(1) A record of thanatosis behaviour in Coronella girondica (Reptilia: Colubridae) Arancha de Castro-Expósito1, Francisco Guerrero1,2 & Enrique García-Muñoz1,3,4,* 1 Departamento de Biología Animal, Biología Vegetal y Ecología. Universidad de Jaén. Campus de las Lagunillas, s/n. 23071 Jaén. Spain. C.e.: [email protected] 2 Centro de Estudios Avanzados en Ciencias de la Tierra (CEACTierra). Universidad de Jaén. Campus de las Lagunillas, s/n. 23071 Jaén. Spain. 3 CESAM, Centro de Estúdios de Ambiente o do Mar. Universidade de Aveiro, Campus Universitário de Santiago. 3810-193 Aveiro. Portugal. 4 CIBIO, Centro de Investigação em Biodiversidade e Recursos Genéticos. Universidade do Porto, Campus Agrário de Vairão. 4485- 661 Vairão. Portugal. Fecha de aceptación: 1 de marzo de 2017. Key words: death feigning, Mediterranean climate, Spain. RESUMEN: La tanatosis o fingimiento de la muerte es un comportamiento de defensa que ha sido previamente descrito en muchas especies. Este es el primer registro de comportamiento de tanatosis en la especie de ofidio Coronella girondica. Dicha observación ha sido realizada en el Parque Natural de las Sierras de Cazorla, Segura y Las Villas, en el sur de España. Thanatosis or tonic immobility is a defen- This paper presents the first record of tha- ce behaviour that appears in some reptile and natosis to our knowledge for the southern amphibian species (Gehlbach, 1970; Toledo et al., smooth snake (Coronella girondica); this being 2011). This behaviour is characterized by the the first graphic evidence for this species (Figu- fact that the individual becomes totally flaccid, re 1). -

A New Miocene-Divergent Lineage of Old World Racer Snake from India
RESEARCH ARTICLE A New Miocene-Divergent Lineage of Old World Racer Snake from India Zeeshan A. Mirza1☯*, Raju Vyas2, Harshil Patel3☯, Jaydeep Maheta4, Rajesh V. Sanap1☯ 1 National Centre for Biological Sciences, Tata Institute of Fundamental Research, Bangalore 560065, India, 2 505, Krishnadeep Towers, Mission Road, Fatehgunj, Vadodra 390002, Gujarat, India, 3 Department of Biosciences, Veer Narmad South Gujarat University, Surat-395007, Gujarat, India, 4 Shree cultural foundation, Ahmedabad 380004, Gujarat, India ☯ These authors contributed equally to this work. * [email protected] Abstract A distinctive early Miocene-divergent lineage of Old world racer snakes is described as a new genus and species based on three specimens collected from the western Indian state of Gujarat. Wallaceophis gen. et. gujaratenesis sp. nov. is a members of a clade of old world racers. The monotypic genus represents a distinct lineage among old world racers is recovered as a sister taxa to Lytorhynchus based on ~3047bp of combined nuclear (cmos) and mitochondrial molecular data (cytb, ND4, 12s, 16s). The snake is distinct morphologi- cally in having a unique dorsal scale reduction formula not reported from any known colubrid snake genus. Uncorrected pairwise sequence divergence for nuclear gene cmos between OPEN ACCESS Wallaceophis gen. et. gujaratenesis sp. nov. other members of the clade containing old Citation: Mirza ZA, Vyas R, Patel H, Maheta J, world racers and whip snake is 21–36%. Sanap RV (2016) A New Miocene-Divergent Lineage of Old World Racer Snake from India. PLoS ONE 11 (3): e0148380. doi:10.1371/journal.pone.0148380 Introduction Editor: Ulrich Joger, State Natural History Museum, GERMANY Colubrid snakes are one of the most speciose among serpents with ~1806 species distributed across the world [1–5]. -

Tail Breakage and Predatory Pressure Upon Two Invasive Snakes (Serpentes: Colubridae) at Two Islands in the Western Mediterranean
Canadian Journal of Zoology Tail breakage and predatory pressure upon two invasive snakes (Serpentes: Colubridae) at two islands in the Western Mediterranean Journal: Canadian Journal of Zoology Manuscript ID cjz-2020-0261.R2 Manuscript Type: Article Date Submitted by the 17-Jan-2021 Author: Complete List of Authors: Febrer-Serra, Maria; University of the Balearic Islands Lassnig, Nil; University of the Balearic Islands Colomar, Victor; Consorci per a la Recuperació de la Fauna de les Illes Balears Draft Sureda Gomila, Antoni; University of the Balearic Islands; Carlos III Health Institute, CIBEROBC Pinya Fernández, Samuel; University of the Balearic Islands, Biology Is your manuscript invited for consideration in a Special Not applicable (regular submission) Issue?: Zamenis scalaris, Hemorrhois hippocrepis, invasive snakes, predatory Keyword: pressure, Balearic Islands, frequency of tail breakage © The Author(s) or their Institution(s) Page 1 of 34 Canadian Journal of Zoology 1 Tail breakage and predatory pressure upon two invasive snakes (Serpentes: 2 Colubridae) at two islands in the Western Mediterranean 3 4 M. Febrer-Serra, N. Lassnig, V. Colomar, A. Sureda, S. Pinya* 5 6 M. Febrer-Serra. Interdisciplinary Ecology Group. University of the Balearic Islands, 7 Ctra. Valldemossa km 7.5, 07122 Palma, Balearic Islands, Spain. E-mail address: 8 [email protected]. 9 N. Lassnig. Interdisciplinary Ecology Group. University of the Balearic Islands, Ctra. 10 Valldemossa km 7.5, 07122 Palma, Balearic Islands, Spain. E-mail address: 11 [email protected]. 12 V. Colomar. Consortium for the RecoveryDraft of Fauna of the Balearic Islands (COFIB). 13 Government of the Balearic Islands, Spain. -

Ancestral Reconstruction of Diet and Fang Condition in the Lamprophiidae: Implications for the Evolution of Venom Systems in Snakes
Journal of Herpetology, Vol. 55, No. 1, 1–10, 2021 Copyright 2021 Society for the Study of Amphibians and Reptiles Ancestral Reconstruction of Diet and Fang Condition in the Lamprophiidae: Implications for the Evolution of Venom Systems in Snakes 1,2 1 1 HIRAL NAIK, MIMMIE M. KGADITSE, AND GRAHAM J. ALEXANDER 1School of Animal, Plant and Environmental Sciences, University of the Witwatersrand, Johannesburg. PO Wits, 2050, Gauteng, South Africa ABSTRACT.—The Colubroidea includes all venomous and some nonvenomous snakes, many of which have extraordinary dental morphology and functional capabilities. It has been proposed that the ancestral condition of the Colubroidea is venomous with tubular fangs. The venom system includes the production of venomous secretions by labial glands in the mouth and usually includes fangs for effective delivery of venom. Despite significant research on the evolution of the venom system in snakes, limited research exists on the driving forces for different fang and dental morphology at a broader phylogenetic scale. We assessed the patterns of fang and dental condition in the Lamprophiidae, a speciose family of advanced snakes within the Colubroidea, and we related fang and dental condition to diet. The Lamprophiidae is the only snake family that includes front-fanged, rear-fanged, and fangless species. We produced an ancestral reconstruction for the family and investigated the pattern of diet and fangs within the clade. We concluded that the ancestral lamprophiid was most likely rear-fanged and that the shift in dental morphology was associated with changes in diet. This pattern indicates that fang loss, and probably venom loss, has occurred multiple times within the Lamprophiidae. -

Observations of Marine Swimming in Malpolon Monspessulanus
Herpetology Notes, volume 14: 593-596 (2021) (published online on 29 March 2021) Snake overboard! Observations of marine swimming in Malpolon monspessulanus Grégory Deso1,*, Xavier Bonnet2, Cornélius De Haan3, Gilles Garnier4, Nicolas Dubos5, and Jean-Marie Ballouard6 The ability to swim is widespread in snakes and not The diversity of fundamentally terrestrial snake restricted to aquatic (freshwater) or marine species species that colonised islands worldwide, including (Jayne, 1985, 1988). A high tolerance to hypernatremia remote ones (e.g., the Galapagos Archipelago), shows (i.e., high concentration of sodium in the blood) enables that these reptiles repeatedly achieved very long and some semi-aquatic freshwater species to use brackish successful trips across the oceans (Thomas, 1997; and saline habitats. In coastal populations, snakes may Martins and Lillywhite, 2019). However, we do not even forage at sea (Tuniyev et al., 2011; Brischoux and know how snakes reached remote islands. Did they Kornilev, 2014). Terrestrial species, however, are rarely actively swim, merely float at the surface, or were observed in the open sea. While snakes are particularly they transported by drifting rafts? We also ignore the resistant to fasting (Secor and Diamond, 2000), long trips mechanisms that enable terrestrial snakes to survive in the marine environment are metabolically demanding during long periods at sea and how long they can do so. (prolonged locomotor efforts entail substantial energy Terrestrial tortoises travel hundreds of kilometres, can expense) and might be risky (Lillywhite, 2014). float for weeks in ocean currents, and can sometimes Limited freshwater availability might compromise the survive in hostile conditions without food and with hydromineral balance of individuals, even in amphibious limited access to freshwater (Gerlach et al., 2006). -

Contents Herpetological Journal
British Herpetological Society Herpetological Journal Volume 31, Number 3, 2021 Contents Full papers Killing them softly: a review on snake translocation and an Australian case study 118-131 Jari Cornelis, Tom Parkin & Philip W. Bateman Potential distribution of the endemic Short-tailed ground agama Calotes minor (Hardwicke & Gray, 132-141 1827) in drylands of the Indian sub-continent Ashish Kumar Jangid, Gandla Chethan Kumar, Chandra Prakash Singh & Monika Böhm Repeated use of high risk nesting areas in the European whip snake, Hierophis viridiflavus 142-150 Xavier Bonnet, Jean-Marie Ballouard, Gopal Billy & Roger Meek The Herpetological Journal is published quarterly by Reproductive characteristics, diet composition and fat reserves of nose-horned vipers (Vipera 151-161 the British Herpetological Society and is issued free to ammodytes) members. Articles are listed in Current Awareness in Marko Anđelković, Sonja Nikolić & Ljiljana Tomović Biological Sciences, Current Contents, Science Citation Index and Zoological Record. Applications to purchase New evidence for distinctiveness of the island-endemic Príncipe giant tree frog (Arthroleptidae: 162-169 copies and/or for details of membership should be made Leptopelis palmatus) to the Hon. Secretary, British Herpetological Society, The Kyle E. Jaynes, Edward A. Myers, Robert C. Drewes & Rayna C. Bell Zoological Society of London, Regent’s Park, London, NW1 4RY, UK. Instructions to authors are printed inside the Description of the tadpole of Cruziohyla calcarifer (Boulenger, 1902) (Amphibia, Anura, 170-176 back cover. All contributions should be addressed to the Phyllomedusidae) Scientific Editor. Andrew R. Gray, Konstantin Taupp, Loic Denès, Franziska Elsner-Gearing & David Bewick A new species of Bent-toed gecko (Squamata: Gekkonidae: Cyrtodactylus Gray, 1827) from the Garo 177-196 Hills, Meghalaya State, north-east India, and discussion of morphological variation for C. -

A Survey on Non-Venomous Snakes in Kashan (Central Iran)
J. Biol. Today's World. 2016 Apr; 5 (4): 65-75 ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ Journal of Biology and Today's World Journal home page: http://journals.lexispublisher.com/jbtw Received: 09 March 2016 • Accepted: 22 April 2016 Research doi:10.15412/J.JBTW.01050402 A survey on Non-Venomous Snakes in Kashan (Central Iran) Rouhullah Dehghani1, Nasrullah Rastegar pouyani2, Bita Dadpour3*, Dan Keyler4, Morteza Panjehshahi5, Mehrdad Jazayeri5, Omid Mehrpour6, Amir Habibi Tamijani7 1 Social Determinants of Health (SDH) Research Center and Department of Environment Health, Kashan University of Medical Sciences, Kashan, Iran 2 Department of Biology, Faculty of Science, Razi University, Kermanshah, Iran 3 Addiction Research Centre, Mashhad University of Medical Sciences, Mashhad, Iran 4 Department of Experimental and Clinical Pharmacology, College of Pharmacy, University of Minnesota, Minneapolis Medical Research Foundation, Minneapolis, MN, United States 5 Health Center of Kashan University Medical Sciences and Health Services, Kashan, Iran 6 Atherosclerosis and Coronary Artery Research Center, Birjand University of Medical Science, Medical Toxicology and Drug Abuse Research Center, Birjand University of Medical Sciences, Birjand, Iran 7 Legal Medicine Research Center, Legal Medicine Organization, Tehran, Iran *Correspondence should be addressed to Bita Dadpour, Addiction Research Centre, Mashhad University of Medical Sciences, Mashhad, Iran; Tell: +985118525315; Fax: +985118525315; Email: [email protected]. ABSTRACT Due to the importance of animal bites in terms of health impacts , potential medical consequences, and the necessity of proper differentiation between venomous and non-venomous snake species, this study was conducted with the aim of identifying non-venomous, or fangless snakes, in Kashan as a major city in central Iran during a three-year period (2010- 2012). -

A Multivariate Analysis of the Morphology of the Colubrid Snake Malpolon Monspessulanus in Morocco and Western Sahara: Biogeographic and Systematic Implications
A multivariate analysis of the morphology of Malpolon monspessulanus SALAMANDRA 42 2/3 65-82 Rheinbach, 20 August 2006 ISSN 0036-3375 A multivariate analysis of the morphology of the colubrid snake Malpolon monspessulanus in Morocco and Western Sahara: biogeographic and systematic implications PHILIPPE GENIEZ, ALEXANDRE CLUCHIER & CORNELIUS C. DE HAAN Abstract. The largely circum-Mediterranean Montpellier Snake Malpolon monspessulanus appears to be highly differentiated in Morocco. Hill & Smith multivariate analyses performed on 68 specimens from south-western Europe and North Africa revealed the existence inside Morocco of three distinct parapatric populations with proper morphological features: (1) the nominal subspecies present in the major part of the country; (2) Malpolon monspessulanus insignitus in the North-East (high plateau), and (3) a new subspecies restricted to the coastal areas of south-western Morocco and Western Sahara. The main features of the new subspecies are 19 rows of dorsal scales at mid-body, with for adult males dorsally a general black pigmentation presenting a small whitish spot on every dorsal scale, while also throat and belly are whitish, longitudinally stained with dark grey, and for adult females dorsally a mostly more pronounced and contrasted expression of the specific, typical female colour pattern and markings, than seen in the females from elsewhere in Morocco. Adult male specimens morphologi- cally intermediate between the new and the nominal subspecies, are recorded in the Souss valley. The Moroccan geographical distribution of the three subspecies is revised. Key words. Squamata, Serpentes, Colubridae, Psammophiinae, Malpolon monspessulanus, systematics, new subspecies, geographical distribution, Morocco, Sahara. Introduction ranean type climate (cf.