Electrophysiological Studies the Antrum Muscle Fibers of the Guinea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

6 Physiology of the Colon : Motility
#6 Physiology of the colon : motility Objectives : ● Parts of the Colon ● Functions of the Colon ● The physiology of Different Colon Regions ● Secretion in the Colon ● Nutrient Digestion in the Colon ● Absorption in the Colon ● Bacterial Action in the Colon ● Motility in the Colon ● Defecation Reflex Doctors’ notes Extra Important Resources: 435 Boys’ & Girls’ slides | Guyton and Hall 12th & 13th edition Editing file [email protected] 1 ﺗﻛرار ﻣن اﻟﮭﺳﺗوﻟوﺟﻲ واﻷﻧﺎﺗوﻣﻲ The large intestine ● This is the final digestive structure. ● It does not contain villi. ● By the time the digested food (chyme) reaches the large intestine, most of the nutrients have been absorbed. ● The primary role of the large intestine is to convert chyme into feces for excretion. Parts of the colon ● The colon has a length of about 150 cm. ( 1.5 meters) (one-fifth of the whole length of GIT). ● It consists of the ascending & descending colon, transverse colon, sigmoid colon, rectum and anal canal. 3 ● The transit of radiolabeled chyme through 4 the large intestine occurs in 36-48 hrs. 2 They know this how? By inserting radioactive chyme. 1 6 5 ❖ Mucous membrane of the colon ● Lacks villi and has many crypts of lieberkuhn. ● They consists of simple short glands lined by mucous-secreting goblet cells. Main colonic secretion is mucous, as the colon lacks digestive enzymes. ● The outer longitudinal muscle layer is modified to form three longitudinal bands called taenia coli visible on the outer surface.(Taenia coli: Three thickened bands of muscles.) ● Since the muscle bands are shorter than the length of the colon, the colonic wall is sacculated and forms haustra.(Haustra: Sacculation of the colon between the taenia.) Guyton corner : mucus in the large intestine protects the intestinal wall against excoriation, but in addition, it provides an adherent medium for holding fecal matter together. -

Anatomy of the Digestive System
The Digestive System Anatomy of the Digestive System We need food for cellular utilization: organs of digestive system form essentially a long !nutrients as building blocks for synthesis continuous tube open at both ends !sugars, etc to break down for energy ! alimentary canal (gastrointestinal tract) most food that we eat cannot be directly used by the mouth!pharynx!esophagus!stomach! body small intestine!large intestine !too large and complex to be absorbed attached to this tube are assorted accessory organs and structures that aid in the digestive processes !chemical composition must be modified to be useable by cells salivary glands teeth digestive system functions to altered the chemical and liver physical composition of food so that it can be gall bladder absorbed and used by the body; ie pancreas mesenteries Functions of Digestive System: The GI tract (digestive system) is located mainly in 1. physical and chemical digestion abdominopelvic cavity 2. absorption surrounded by serous membrane = visceral peritoneum 3. collect & eliminate nonuseable components of food this serous membrane is continuous with parietal peritoneum and extends between digestive organs as mesenteries ! hold organs in place, prevent tangling Human Anatomy & Physiology: Digestive System; Ziser Lecture Notes, 2014.4 1 Human Anatomy & Physiology: Digestive System; Ziser Lecture Notes, 2014.4 2 is suspended from rear of soft palate The wall of the alimentary canal consists of 4 layers: blocks nasal passages when swallowing outer serosa: tongue visceral peritoneum, -
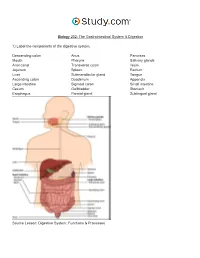
The Gastrointestinal System & Digestion Visual Worksheet
Biology 202: The Gastrointestinal System & Digestion 1) Label the components of the digestive system. Descending colon Anus Pancreas Mouth Pharynx Salivary glands Anal canal Transverse colon Ileum Jejunum Spleen Rectum Liver Submandibular gland Tongue Ascending colon Duodenum Appendix Large intestine Sigmoid colon Small intestine Cecum Gallbladder Stomach Esophagus Parotid gland Sublingual gland Source Lesson: Digestive System: Functions & Processes 2) Label the image below. Serosa Submucous plexus Muscularis externa Submucosa Myenteric plexus Muscular interna Source Lesson: Role of the Enteric Nervous System in Digestion 3) Label the structures of the alimentary canal. Some terms may be used more than once. Vein Mesentery Mucosa Submucosal plexus Epithelium Gland in mucosa Nerve Muscularis Serosa Lymphatic tissue Muscularis mucosae Glands in submucosa Lamina propria Submucosa Duct of gland outside tract Gland in mucosa Lumen Artery Longitudinal muscle Musculararis Areolar connective tissue Circular muscle Myenteric plexus Source Lesson: The Upper Alimentary Canal: Key Structures, Digestive Processes & Food Propulsion 4) Label the image below. Stomach Trachea Lower esophageal sphincter Esophagus Upper esophageal sphincter Source Lesson: The Upper Alimentary Canal: Key Structures, Digestive Processes & Food Propulsion 5) Label the anatomy of the oral cavity. Upper lip Tonsil Inferior labial frenulum Floor of mouth Tongue Superior labial frenulum Lower lip Teeth Retromolar trigone Palatine arch Hard palate Uvula Glossopalatine arch Soft palate Gingiva Source Lesson: The Oral Cavity: Structures & Functions 6) Label the structures of the oral cavity. Some terms may be used more than once. Hard palate Oropharynx Soft palate Pharyngeal tonsil Oral cavity Lingual tonsil Superior lip Teeth Palatine tonsil Tongue Inferior lip Source Lesson: The Oral Cavity: Structures & Functions 7) Label the image below. -

Internal Anal Sphincter
Arch Dis Child: first published as 10.1136/adc.43.231.569 on 1 October 1968. Downloaded from Arch. Dis. Childh., 1968, 43, 569. Internal Anal Sphincter Observations on Development and Mechanism of Inhibitory Responses in Premature Infants and Children with Hirschsprung's Disease E. R. HOWARD and H. H. NIXON From The Hospitalfor Sick Children, Great Ormond Street, London W.C.1 The relative importance of the internal and obstruction to constipation alone. During this external sphincters to the maintenance of tone in study physiological abnormalities were observed in the anal canal has been shown in previous studies of the reflexes of premature infants, which showed anal physiology (Gaston, 1948; Schuster et al., 1965; similarities to those seen in patients with Hirsch- Duthie and Watts, 1965). sprung's disease. On repeated examinations over The external sphincter is a striated muscle, but several days, however, the physiological responses shows continuous activity on electromyography. were found to change until normal reflexes were Inhibition and stimulation is mediated by spinal eventually established. cord reflexes, through the pudendal nerves and In order to help determine the nervous pathway sacral segments of the spinal cord (Floyd and Walls, through which the reflexes of the internal sphincter copyright. 1953; Porter, 1961). Voluntary control is possible are mediated, we have examined normal bowel over this part ofthe anal sphincter. and aganglionic bowel from cases of Hirschsprung's The internal sphincter is made up of smooth disease by pharmacological and histochemical muscle fibres, continuous with the muscle layers of methods. the rectal wall, and under resting conditions pro- vides most of the tone of the anal canal (Duthie and Physiological Study Watts, 1965). -

Aandp2ch25lecture.Pdf
Chapter 25 Lecture Outline See separate PowerPoint slides for all figures and tables pre- inserted into PowerPoint without notes. Copyright © McGraw-Hill Education. Permission required for reproduction or display. 1 Introduction • Most nutrients we eat cannot be used in existing form – Must be broken down into smaller components before body can make use of them • Digestive system—acts as a disassembly line – To break down nutrients into forms that can be used by the body – To absorb them so they can be distributed to the tissues • Gastroenterology—the study of the digestive tract and the diagnosis and treatment of its disorders 25-2 General Anatomy and Digestive Processes • Expected Learning Outcomes – List the functions and major physiological processes of the digestive system. – Distinguish between mechanical and chemical digestion. – Describe the basic chemical process underlying all chemical digestion, and name the major substrates and products of this process. 25-3 General Anatomy and Digestive Processes (Continued) – List the regions of the digestive tract and the accessory organs of the digestive system. – Identify the layers of the digestive tract and describe its relationship to the peritoneum. – Describe the general neural and chemical controls over digestive function. 25-4 Digestive Function • Digestive system—organ system that processes food, extracts nutrients, and eliminates residue • Five stages of digestion – Ingestion: selective intake of food – Digestion: mechanical and chemical breakdown of food into a form usable by -
Bacteria Slides
BACTERIA SLIDES Cocci Bacillus BACTERIA SLIDES _______________ __ BACTERIA SLIDES Spirilla BACTERIA SLIDES ___________________ _____ BACTERIA SLIDES Bacillus BACTERIA SLIDES ________________ _ LUNG SLIDE Bronchiole Lumen Alveolar Sac Alveoli Alveolar Duct LUNG SLIDE SAGITTAL SECTION OF HUMAN HEAD MODEL Superior Concha Auditory Tube Middle Concha Opening Inferior Concha Nasal Cavity Internal Nare External Nare Hard Palate Pharyngeal Oral Cavity Tonsils Tongue Nasopharynx Soft Palate Oropharynx Uvula Laryngopharynx Palatine Tonsils Lingual Tonsils Epiglottis False Vocal Cords True Vocal Cords Esophagus Thyroid Cartilage Trachea Cricoid Cartilage SAGITTAL SECTION OF HUMAN HEAD MODEL LARYNX MODEL Side View Anterior View Hyoid Bone Superior Horn Thyroid Cartilage Inferior Horn Thyroid Gland Cricoid Cartilage Trachea Tracheal Rings LARYNX MODEL Posterior View Epiglottis Hyoid Bone Vocal Cords Epiglottis Corniculate Cartilage Arytenoid Cartilage Cricoid Cartilage Thyroid Gland Parathyroid Glands LARYNX MODEL Side View Anterior View ____________ _ ____________ _______ ______________ _____ _____________ ____________________ _____ ______________ _____ _________ _________ ____________ _______ LARYNX MODEL Posterior View HUMAN HEART & LUNGS MODEL Larynx Tracheal Rings Found on the Trachea Left Superior Lobe Left Inferior Lobe Heart Right Superior Lobe Right Middle Lobe Right Inferior Lobe Diaphragm HUMAN HEART & LUNGS MODEL Hilum (curvature where blood vessels enter lungs) Carina Pulmonary Arteries (Blue) Pulmonary Veins (Red) Bronchioles Apex (points -

Morphology of the Gastrointestinal Tract in Primates : Comparisons with Other Mammals in Relation to Diet David J Chivers, Claude Marcel Hladik
Morphology of the gastrointestinal tract in primates : Comparisons with other mammals in relation to diet David J Chivers, Claude Marcel Hladik To cite this version: David J Chivers, Claude Marcel Hladik. Morphology of the gastrointestinal tract in primates : Com- parisons with other mammals in relation to diet. Journal of Morphology, Wiley, 1980, 166, pp.337-386. hal-00561758 HAL Id: hal-00561758 https://hal.archives-ouvertes.fr/hal-00561758 Submitted on 16 Mar 2013 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. CHIVERS D.J. & HLADIK C.M. (1980) — Morphology of the gastrointestinal tract in primates : Comparisons with other mammals in relation to diet. Journal of Morphology, 166 : 337-386. Flattened pieces of intestinal tract in a dissecting tray, for the actual measurement of mucosal area. 338 DAVID J. CliiVERS AND C. M. HLADIK G 'I' ~IOI!PIIOI.OGY Ai\llDIET I ~li\~ 1\ I AI.S 339 '67) showed interesting relationsh ips among available in limited qua ntity (fruit), to t hose reductions are clearly specializations, rather a "muscular tooth" compensating fo r the lack primates, but data on diet were st ill inade that are widely abundant bui relatively diffi than representing the prim it ive condition. -

Human Colonic Smooth Muscle: Spontaneous Contractile Activity and Response to Stretch
Gut: first published as 10.1136/gut.27.9.1006 on 1 September 1986. Downloaded from Gut, 1986, 27, 1006-1013 Human colonic smooth muscle: spontaneous contractile activity and response to stretch RtC GILL, K R COTE, K L BOWES, AND Y J KINGMA From the Departments ofSurgery and Electrical Engineering, University ofAlberta, Edmonton, Alberta, Canada SUMMARY The length dependence of the spontaneous contractile activity of human colonic muscle was assessed in vitro. Muscle obtained from the right colon was more distensible than that of the left colon. This was true for all muscle layers. Maximum spontaneous active stress was exerted by both circular and longitudinal muscle layers of the right colon at greater degrees of stretch (p<0-001) than those of the left colon. The contractile frequency of longitudinally oriented strips increased with length. The contractile frequency of intertaenial longitudinally oriented strips from the right colon was lower (p<0001) than that of strips from the left colon. The contractile frequency of circularly-oriented strips from the right colon (6.25+±038 min) was higher (p<0-001) than that of strips from the left colon (3-35±0-35 min). The human colon appears to consist of two distinct areas based on the mechanical behaviour of the smooth muscle during spontaneous contraction. The human large intestine may be subdivided into muscle obtained from several animal species has right and left parts on the basis of differing been reported although little is known concerning blood supply, innervation and the dependence of these spontaneous contractions embryology, http://gut.bmj.com/ function.1 The right colon has a greater diameter upon muscle length. -

Anatomy of the Caecum, Appendix and Colon Is the Branches of the Middle and Left Colic Vessels, Resulting in Described
BASIC SCIENCE colon. The embryonic gut then twists to the right (ascending Anatomy of the caecum, colon) and then to the left (descending colon) so these parts become retroperitoneal. It drags its blood supply with it which appendix and colon explains why the right colon is supplied by branches of the superior mesenteric artery and the left colon by the inferior Harold Ellis mesenteric artery. Surgical mobilization of the colon follows these tissue planes to restore its midline position, thus the safe approach on each side is from lateral to medial. There is Abstract a natural vascular watershed in the transverse colon between The gross and microscopic anatomy of the caecum, appendix and colon is the branches of the middle and left colic vessels, resulting in described. An embryological explanation of the adult form is included. the splenic flexure being particularly vulnerable to ischaemia. There is also a note on cancer spread. Peritoneal attachments Keywords Anatomy; appendix; ascending colon; blood supply; caecum; The transverse and sigmoid colon are completely peritonealized, descending colon; lymphatic drainage; sigmoid colon; transverse colon hanging onto the transverse and the sigmoid mesocolon respec- tively. The transverse colon is readily identified by its attachment, along its free border, to the greater omentum. In contrast, the ascending and descending colons adhere to the peritoneum of the The large bowel is subdivided for descriptive purposes into: the posterior abdominal wall. This adhesion is avascular, and enables caecum and appendix, the ascending colon, hepatic flexure, the surgeon easily to mobilize these parts of the large bowel. The transverse colon, splenic flexure, descending and sigmoid colon caecum is usually completely peritonealized, as may occasionally and the rectum and anal canal (Figure 1). -
Digestive System
Chapter 25 *Lecture PowerPoint The Digestive System *See separate FlexArt PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Introduction • Most nutrients we eat cannot be used in existing form – Must be broken down into smaller components before the body can make use of them • Digestive system—essentially a disassembly line – To break down nutrients into a form that can be used by the body – To absorb them so they can be distributed to the tissues • Gastroenterology—the study of the digestive tract and the diagnosis and treatment of its disorders 25-2 General Anatomy and Digestive Processes • Expected Learning Outcomes – List the functions and major physiological processes of the digestive system. – Distinguish between mechanical and chemical digestion. – Describe the basic chemical process underlying all chemical digestion, and name the major substrates and products of this process. 25-3 General Anatomy and Digestive Processes Cont. – List the regions of the digestive tract and the accessory organs of the digestive system. – Identify the layers of the digestive tract and describe its relationship to the peritoneum. – Describe the general neural and chemical controls over digestive function. 25-4 Digestive Function • Digestive system—the organ system that processes food, extracts nutrients from it, and eliminates the residue 25-5 Digestive Function • Five stages of digestion – Ingestion: selective intake of -

Sorenson Atlas of Human Histology Chapters-1-And-14
Atlas of Human Histology A Guide to Microscopic Structure of SAMPLECells, Tissues and Organs Robert L. Sorenson SAMPLE TABLE OF CONTENTS CHAPTER 1 INTRODUCTION AND CELL 1 CHAPTER 2 EPITHELIUM 15 CHAPTER 3 CONNECTIVE TISSUE 29 CHAPTER 4 MUSCLE TISSUE 43 CHAPTER 5 CARTILAGE AND BONE 61 CHAPTER 6 NERVE TISSUE 85 CSAMPLEHAPTER 7 PERIPHERAL BLOOD 107 CHAPTER 8 HEMATOPOESIS 113 CHAPTER 9 CARDIOVASCULAR SYSTEM 127 CHAPTER 10 LYMPHOID SYSTEM 157 CHAPTER 11 SKIN 181 CHAPTER 12 EXOCRINE GLANDS 193 CHAPTER 13 ENDOCRINE GLANDS 205 CHAPTER 14 GASTROINTESTINAL TRACT 223 CHAPTER 15 LIVER AND GALL BLADDER 247 CHAPTER 16 URINARY SYSTEM 261 CHAPTER 17 RESPIRATORY SYSTEM 289 CHAPTER 18 FEMALE REPRODUCTIVE SYSTEM 305 CHAPTER 19 MALE REPRODUCTIVE SYSTEM 329 CHAPTER 20 ORGANS OF SPECIAL SENSE 343 INDEX 359 i This atlas is a series of photographs ranging from low to high magnifications of the indi- vidual tissue specimens. The low magnification images should be used for orientation, while the higher magnification images show details of cells, tissues, and organs. Al - though every effort has been made to faithfully reproduce the colors of the tissues, a full appreciation of histological structure is best achieved by examining the original speci- mens with a microscope. This atlas is a preview of what should be observed. The photomicrographs found in this atlas come from the collection of microscope slide used by medical, dental and undergraduate students of histology at the University of Minnesota. Most of these slides were prepared by Anna-Mary Carpenter M.D., Ph.D. during her tenure as Professor in the Department of Anatomy (University of Minnesota MedicalSAMPLE School). -

Organization of the Muscular Wall Ofthe Human Colon
Gut, 1969, 10, 352-359 Gut: first published as 10.1136/gut.10.5.352 on 1 May 1969. Downloaded from Organization of the muscular wall ofthe human colon J. L. PACE AND IFOR WILLIAMS1 From the Department ofAnatomy, Royal University of Malta, and the Research Department, St Mark's Hospital, London The colon is the portion of the alimentary tract that ascending colon, and 20 from the transverse colon. All has generated least interest among investigators and areas, including the three taeniae, were examined at some although the last decade has seen new methods time both distended and opened before fixation. Those applied to physiological problems in the large bowel, specimens with visible diverticula were excluded, but in view of the age of the patients who have part of the colon the detailed anatomy of the muscular layer remains removed for neoplasm, and these were the bulk, it is a neglected subject, with the successfully located likely that some examples of diverticular disease escaped articles which consider this matter very few in detection (Williams, 1967). No muscle was obviously number. hypertrophied, but here again the impossibility of Each of us undertook an independent investigation diagnosis of this in life, lack of knowledge of how long and in this field of pattern recognition where it takes to regress after a colostomy, and the great observer error can be very gross, our conclusions difficulty of diagnosing it in the specimen, means that a have been surprisingly similar, so much so that a few examples may have gone undetected. It is an account common of the mature and ageing colon.