211.Full.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

First Report on the State of the World's Animal Genetic Resources"
"First Report on the State of the World’s Animal Genetic Resources" (SoWAnGR) Country Report of the United Kingdom to the FAO Prepared by the National Consultative Committee appointed by the Department for Environment, Food and Rural Affairs (Defra). Contents: Executive Summary List of NCC Members 1 Assessing the state of agricultural biodiversity in the farm animal sector in the UK 1.1. Overview of UK agriculture. 1.2. Assessing the state of conservation of farm animal biological diversity. 1.3. Assessing the state of utilisation of farm animal genetic resources. 1.4. Identifying the major features and critical areas of AnGR conservation and utilisation. 1.5. Assessment of Animal Genetic Resources in the UK’s Overseas Territories 2. Analysing the changing demands on national livestock production & their implications for future national policies, strategies & programmes related to AnGR. 2.1. Reviewing past policies, strategies, programmes and management practices (as related to AnGR). 2.2. Analysing future demands and trends. 2.3. Discussion of alternative strategies in the conservation, use and development of AnGR. 2.4. Outlining future national policy, strategy and management plans for the conservation, use and development of AnGR. 3. Reviewing the state of national capacities & assessing future capacity building requirements. 3.1. Assessment of national capacities 4. Identifying national priorities for the conservation and utilisation of AnGR. 4.1. National cross-cutting priorities 4.2. National priorities among animal species, breeds, -

Copyrighted Material
FTOC 08/27/2018 10:24:30 Page v Contents Acknowledgements ix Introduction 1 Standard feather markings 4 Chief points of the fowl 13 Complete classification of pure breed poultry 21 Defects and deformities 25 Large fowl and bantams 31 Ancona 31 Andalusian 34 Appenzeller 36 Araucana 41 Rumpless Araucana 45 Asil 48 Australorp 50 Autosexing breeds 53 Brockbar 54 Brussbar 55 Cambar 57 Dorbar 59 Legbar 60 Rhodebar 63 Welbar 65 Wybar 68 Ayam Cemani 71 Barnevelder 72 Belgian Bearded bantamsCOPYRIGHTED MATERIAL 75 Barbu d’Anvers 75 Barbu d’Uccle 77 Barbu de Watermael 78 Barbu d’Everberg (Rumpless d’Uccle) 80 Barbu du Grubbe (Rumpless d’Anvers) 81 Barbu de Boitsfort (Rumpless de Watermael) 81 Booted bantam 87 Rumpless Booted Bantam 92 Brabanter 93 Brahma 95 Brakel 100 FTOC 08/27/2018 10:24:30 Page vi vi Contents Breda 102 Bresse-Gauloise 104 Burmese 106 Campine 108 Cochin 111 Crèvecoeur 114 Croad Langshan 115 Dandarawi 118 Derbyshire Redcap 120 Dominique 121 Dorking 124 Dutch bantam 128 British Faverolles 133 Fayoumi 137 Friesian 139 Frizzle 143 German Langshan 145 Groninger 149 Hamburgh 152 Houdan 155 Indian Game 158 Ixworth 162 Japanese bantam 164 Jersey Giant 169 Ko Shamo 171 Kraienköppe 173 Kulang 177 La Flèche 179 Lakenvelder 181 Leghorn 183 Lincolnshire Buff 188 Malay 191 Marans 194 Marsh Daisy 198 Minorca 201 Modern Game 204 Modern Langshan 209 Nankin bantam 212 Nankin Shamo 214 New Hampshire Red 215 Norfolk Grey 218 North Holland Blue 220 Ohiki 222 Carlisle Old English Game 223 Oxford Old English Game 230 Old English Game bantam 236 Old English -

Poultry in the United Kingdom the Genetic Resources of the National Flocks
www.defra.gov.uk Poultry in the United Kingdom The Genetic Resources of the National Flocks November 2010 Cover: Red Dorking male (photograph John Ballard, courtesy of The Cobthorn Trust) All information contained in this brochure was correct at time of going to press (December 2010). Department for Environment, Food and Rural Affairs Nobel House 17 Smith Square London SW1P 3JR Telephone: 020 7238 6000 Website: www.defra.gov.uk © Crown copyright 2010 Copyright in the typographical arrangement and design rests with the Crown. This publication (excluding the logo) may be reproduced free of charge in any format or medium provided that it is reproduced accurately and not used in a misleading context. The material must be acknowledged as Crown copyright with the title and source of the publication specified. This document is also available on the Defra website. Published by the Department for Environment, Food and Rural Affairs. Printed in the UK, December 2010, on material that contains a minimum of 100% recycled fibre for uncoated paper and 75% recycled fibre for coated paper. PB13451 December 2010. Contents 1. Introduction 3 2. Poultry keeping systems 4 3. Species Accounts 6 3.1. The Domestic Fowl (Gallus gallus domesticus) 6 3.2. Turkeys 8 3.3. Ducks 8 3.4. Geese 9 3.5. Minor Species 9 4. Breed Organisations 10 5. Data Recording and Registration 11 6. References 12 7. Annex: Current situation for individual breeds and strains 13 Abbreviations 24 1 1. Introduction Domestic poultry form the most important sector of livestock keeping worldwide, the production of meat and eggs being a major contributor to human nutrition. -

British Poultry Standards
British Poultry Standards Complete specifi cations and judging points of all standardized breeds and varieties of poultry as compiled by the specialist Breed Clubs and recognised by the Poultry Club of Great Britain Sixth Edition Edited by Victoria Roberts BVSc MRCVS Honorary Veterinary Surgeon to the Poultry Club of Great Britain Council Member, Poultry Club of Great Britain This edition fi rst published 2008 © 2008 Poultry Club of Great Britain Blackwell Publishing was acquired by John Wiley & Sons in February 2007. Blackwell’s publishing programme has been merged with Wiley’s global Scientifi c, Technical, and Medical business to form Wiley-Blackwell. Registered offi ce John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, United Kingdom Editorial offi ce 9600 Garsington Road, Oxford, OX4 2DQ, United Kingdom For details of our global editorial offi ces, for customer services and for information about how to apply for permission to reuse the copyright material in this book please see our website at www.wiley.com/wiley-blackwell. The right of the author to be identifi ed as the author of this work has been asserted in accordance with the Copyright, Designs and Patents Act 1988. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher. Wiley also publishes its books in a variety of electronic formats. Some content that appears in print may not be available in electronic books. -

RARE POULTRY CLASSES 8 Malay Pullet
ASIAN HARDFEATHER 36 Ko Shamo Black/White/Self Pullet Regional Show 37 Ko Shamo AOC Male Sec: Z Shakeshaft Tel: 07866 575316 38 Ko Shamo AOC Female Judges : Mr R Francis (Large Fowl) 39 Ko Shamo AOC Stag & Mr P Cox ( Bantams) 40 Ko Shamo AOC Pullet 0 41 Asil Male LARGE FOWL 42 Asil Female 43 Tuzo Male 1 Asil Male 44 Tuzo Female 2 Asil Female 45 Non Standard/AOV Male 3 Asil Stag 46 Non Standard/AOV Female 4 Asil Pullet See Juvenile section for entries 5 Malay Male 6 Malay Female 7 Malay Stag RARE POULTRY CLASSES 8 Malay Pullet 9 O Shamo Male (8.8lbs+) RARE POULTRY SOCIETY 10 O Shamo Female (6.6lbs+) Club Show 11 Chu Shamo Male (Under 8.8lbs) Sec: P M Fieldhouse Tel: 01934 12 Chu Shamo Female (under 6.6lbs) 824213 (eve) 13 Shamo Stag (Any size) Judges: Mr J Lockwood 14 Shamo Pullet (Any Size) (Hardfeather & Soft Feather Heavy) 15 Kulang Male Mr P Hayford (True Bantams, 16 Kulang Female Longtails, Juveniles, Pairs & Trios) 17 Satsumadori Male Mr A Hillary (Soft Feather Light 18 Satsumadori Female Breeds 1) 19 Yamato Gunkei Male Mr J Robertson (Soft Feather Light 20 Yamato Gunkei Female Breeds 2) 21 Yamato Gunkei Stag 22 Yamato Gunkei Pullet LONG TAILED LARGE 23 Non Standard/AOV Male 24 Non Standard/AOV Female 47 Yokohama Male See Juvenile section for entries 48 Sumatra Male 49 Yokohama Female BANTAMS 50 Sumatra Female 25 Malay Male LARGE SOFTFEATHER LIGHT 26 Malay Female BREEDS 27 Ko Shamo Black Red Male 28 Ko Shamo Black Red Stag 51 Andalusian Male 29 Ko Shamo Duckwing Male 52 Andalusian Female 30 Ko Shamo Duckwing Stag 53 Ayam Cemani M/F -
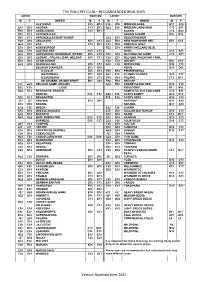
Ringing Scheme Ring Sizes
THE POULTRY CLUB – RECOMMENDED RING SIZES LARGE BANTAM LARGE BANTAM M F BREED M F M F BREED M F ALSTEIRER K15 M12 E18 D16 MODERN GAME B11 A10 E18 D16 ANCONA C13 B11 G22 E18 MODERN LANGSHAN C13 B11 E18 K15 ANDALUSIAN C13 B11 NANKIN C13 M12 D16 C13 APPENZELLER NANKIN SHAMO D16 D16 D16 C13 APPENZELLER BARTHUNER G22 E18 NEIDERRHEINER F20 D16 ARAUCANA K15 C13 G22 E18 NEW HAMPSHIRE RED K15 C13 G22 E18 ASIL C13 B11 E18 D16 NORFOLK GREY E18 D16 AUGSBURGER G22 E18 NORTH HOLLAND BLUE G22 E18 AUSTRALORP K15 C13 OHIKI C13 B11 G22 E18 AUTOSEXING: RHODEBAR, WYBAR K15 C13 E18 D16 OLD ENGLISH GAME C13 B11 E18 D16 LEGBAR , CREAM L’BAR, WELBAR K15 C13 F20 E18 OLD ENG. PHEASANT FOWL D16 D16 AYAM CEMANI F20 E18 ORLOFF D16 C13 G22 E18 BARNEVELDER K15 C13 G22 F20 ORPINGTON D16 C13 BELGIAN D’ANVERS B11 A10 PEKIN D16 D16 D’UCCLE D16 C13 F20 E18 PENEDESENCA WATERMAEL B11 A10 G22 E18 PLYMOUTH ROCK K15 C13 D’EVERBURG D16 C13 D16 D16 POLAND C13 B11 DE GRUBBE, DE BOITSFORT B11 A10 F20 E18 REDCAP J27 G22 BELGIAN GAME: BRUGES G22 E18 RHODE ISLAND RED K15 C13 G22 E18 LIĖGE ROSECOMB B11 A10 G22 C13 BERGISCHE KRAHER RUMPLESS OLD ENG GAME C13 B11 BOOTED D16 C13 G22 F20 SCOTS DUMPY D16 C13 D16 C13 BRABANTER F20 E18 SCOTS GREY K15 C13 J27 J27 BRAHMA E18 D16 SEBRIGHT B11 A10 E18 D16 BRAKEL SERAMA B11 A10 G22 F20 BREDA G22 F20 SHAMO E18 D16 BRESSE-GAULOIS E18 D16 SICILIAN BUTTERCUP D16 C13 G22 E18 BUCKEYE E18 D16 SILKIE C13 B11 H24 G22 BUFF ORPINGTON K15 C13 E18 D16 SPANISH K15 C13 BURMESE D16 C13 G22 F20 SULMTALER D16 C13 D16 C13 CAMPINE F20 E18 SULTAN J27 J27 COCHIN E18 -
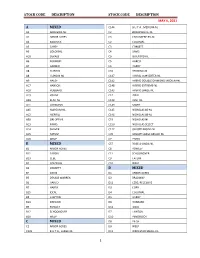
Stock Codes Alphabetical
STOCK CODE DESCRIPTION STOCK CODE DESCRIPTION MAY 6, 2021 A MIXED C144 B.U.T.A., MEDIUM-NL A2 ANDREWS-NL C2 BROADWHITE-NL A1 ARBOR ACRES C3 CHAUMIERE BB-NL A2 BABCOCK C2 COLONIAL A3 CAREY C3 CORBETT A5 COLONIAL C4 DAVIS A18 DEKALB C9 GUILFORD-NL A6 EURIBRID C5 HARCO A7 GARBER C6 HARDY A8 H AND N C10 HENNING-NL A8 H AND N-NL C147 HYBRID CONVERTER-NL A9 HALEY C142 HYBRID DOUBLE DIAMOND MEDIUM-NL A17 HANSON C148 HYBRID EXTREME-NL A10 HUBBARD C143 HYBRID LARGE-NL A19 HYLINE C12 IDEAL A38 KENT-NL C129 KENT-NL A11 LOHMANN C149 MIXED A45 MARCUM-NL C145 NICHOLAS 85-NL A12 MERRILL C146 NICHOLAS 88-NL A58 ORLOPP-NL C19 NICHOLAS-NL A13 PARKS C150 NICHOLAS SELECT A14 SHAVER C122 ORLOPP BROAD-NL A15 TATUM C35 ORLOPP LARGE BROAD-NL A16 WELP C7 PARKS B MIXED C57 ROSE-A-LINDA-NL B1 ARBOR ACRES C8 ROWLEY B11 CARGILL C11 SCHOONOVER B13 CEBE C9 TATUM B2 COLONIAL C10 WELP B3 CORBETT D MIXED B4 DAVIS D1 ARBOR ACRES B5 DEKALB WARREN D2 BRADWAY B6 HARCO D11 CEBE, RECESSIVE B7 HARDY D3 COBB B15 IDEAL D4 COLONIAL B8 LAWTON D5 HARDY B14 OREGON D6 HUBBARD B9 ROWLEY D12 IDEAL B12 SCHOONOVER D7 LAWTON B10 WELP D10 PENOBSCOT C MIXED D8 PILCH C1 ARBOR ACRES D9 WELP C135 B.U.T.A., LARGE-NL D11 WROLSTAD SMALL-NL 1 STOCK CODE DESCRIPTION STOCK CODE DESCRIPTION MAY 6, 2021 N36 BIGHAM RED E MIXED N202 BILL ROBERT'S BUTCHER E3 BOURBON, RED-NL N287 BLACK E6 CEBE N253 BLACK, DENT COUNTY E1 COLONIAL N266 BLUE E2 HUBBARD N240 BLUE FACE E3 ROWLEY N112 BLUE SPLASH E5 SCHOONOVER N242 BONANZA BLACK E4 WELP N40 BRISTER CHICK H MIXED N90 BROWN RED H1 ARBOR ACRES N260 BROWN RED HATCH, -

David Scrivener Collection D DS
Museum of English Rural Life David Scrivener Collection D DS David Scrivener (1952 – 2015) was a committed and enthusiastic poultry- keeper. He earned a National Diploma in Poultry from Harper Adams Agricultural College in Shropshire. He utilised his expertise as a judge at various shows for the Poultry Club of Great Britain. David had a passion for rare breeds, particularly the Spanish, which he kept, and fulfilled various roles for the Rare Poultry Society, variously acting as Chairman, Honorary Historian and Patron. He was the author of several books on the subject including Starting with Bantams (2002), Exhibition Poultry Keeping (2005), Rare Poultry Breeds (2006) and Popular Poultry Breeds (2009). The Collection covers the year’s 1893 to 2007; undated. The physical extent of the collection is 17 boxes, 13 oversize items. D DS 1 Slides c. 1980 to 2015 Contains slides of various poultry species arranged by species and breed. Much of the information used here was obtained from Scrivener’s own annotations for each slide. Many of these photographs are studio portraits, used to illustrate Scrivener’s publications. A large proportion of the photographs were taken by John Tarren. Some boxes have been labelled with people’s names, presumably friends and fellow poultry breeders. 8 boxes Page 1 of 279 Museum of English Rural Life D DS 1/1 American Old English Game Box 1 c.1980 to 2015 Contains colour slides featuring photographs of various American Old English Game chickens. These images were all taken indoors, in some form of photographic studio. 6 slides D DS 1/1/1 Slide of a male American Old English Game chicken c.1980 to 2015 Studio photograph 1 slide D DS 1/1/2 Slide of an American Old English Game chicken c.1980 to 2015 Studio photograph, labelled AF. -

Large Breeds, Bantam Breeds and Eggs
STANDARD SHOW CODES The various codes contained in this standard show codes form part of a computer programme used by show administrators to organise and run poultry shows. It is very important that all the information furnished on the entry form should be correct in all aspects. The programme is of such a nature that we cannot correct mistakes after the programme has been put into operation. STANDARD POULTRY BREEDS The standard show codes contain lists of all the different types of poultry that possibly exist. Each breed has its own distinctive code number and the various breeds are numbered as follows: (1) Chicken breeds 100 – 699 (2) Turkey breeds 700 – 749 (3) Goose breeds 750 – 799 (4) Duck breeds 800 – 899 (5) Eggs 970 – 999 COLOUR CHART On the pages marked "COLOUR CHART" are lists of all the poultry colours that possibly exist and each colour has a code number which applies to large breeds, bantam breeds and eggs. The colours applicable to the various breeds are fully described and coded in the Breed Standards and are the only colours recognised which should be used when entering birds or eggs. The colour codes for OEG are separately listed and can be found at the end of the colour chart. Please note that the information furnished in the MORE INFO column should not be entered on the entry form or the computer programme. In order to assist exhibitors the colour codes are included in the Breed Standards. Please visit www.poultryclubsa.co.za and click on Breed Standards. CLASSIFICATION On the page marked "CLASSIFICATION" is a list of sexes of birds and this applies to all the poultry breeds. -

Genetic Analysis of Production Traits to Support Breeding Programs Utilizing Indigenous Chickens in Ethiopia
Breeding programs for indigenous chicken in Ethiopia Analysis of diversity in production systems and chicken populations Nigussie Dana 1 Thesis Committee Thesis supervisor Prof. dr. ir. J.A.M. van Arendonk Professor of Animal Breeding and Genetics Wageningen University, The Netherlands Thesis co-supervisors Dr. ir. E.H. van der Waaij Assistant professor Animal Breeding and Genetics Wageningen University, The Netherlands Dr. T. Dessie Research officer International Livestock Research Institute, Ethiopia Other Members Prof. dr. J. Sölkner BOKU University, Vienna (Austria) Prof. dr. M. C. H. de Jong Wageningen University (The Netherlands) Prof. dr. A.K. Kahi Egerton University (Kenya) Dr. G. A.A. Albers Hendrix Genetics, Boxmeer (The Netherlands) This research was conducted under the auspices of the Graduate School of Wageningen Institute of Animal Sciences (WIAS). 2 Breeding programs for indigenous chicken in Ethiopia Analysis of diversity in production systems and chicken populations Nigussie Dana Thesis Submitted in fulfilment of the requirements for the degree of doctor at Wageningen University by the authority of the Rector Magnificus, Prof. dr. M.J. Kropff, in the presence of the Thesis Committee appointed by the Academic Board to be defended in public on Friday 4 February 2011 at 4 p.m. in the Aula 3 Nigussie Dana Breeding programs for indigenous chicken in Ethiopia: analysis of diversity in production systems and chicken populations, 148 pages. PhD thesis, Wageningen University, The Netherlands (2011) With summary in English and Dutch ISBN: 978-90-8585-872-0 4 Abstract The aim of this research was to generate information required to establish a sustainable breeding program for improving the productivity of locally adapted chickens to enhance the livelihood of rural farmers in Ethiopia. -

Grosshühner Grandes Volailles Large Fowl
GROSSHÜHNER GRANDES VOLAILLES LARGE FOWL Altenglische Kämpfer Combattant anglais ancien Old-English game Altsteirer Alsteirer Alsteirer Amerikanische Leghorn Leghorn américaine American Leghorn Amrock Amrock Amrock Ancona Ancône Ancona Andalusier Andalouse Andalusian Appenzeller Spitzhauben Appenzelloise huppée Appenzeller Spitzhauben Araucana (Amerikanische Selektion) Araucana (sélection américaine) Rumpless Araucana (american selection) Araucana (Deutsche Selektion) Araucana (sélection allemande) Rumpless Araucana (german selection) Ardenner Ardennaise Ardenner Ardenner Kaulhühner Sans-queue des Ardennes Rumpless Ardenner Asil Asyl Asyl Augsburger Augsbourg Augsburg Australorps Australorp Australorp Barnevelder Barnevelder Barnevelder Bergische Kräher Chanteur du Berg Berg crower Bielefelder Kennhühner Poule de Bielefeld Bielefelder Brahma Brahma Brahma Brakel Braekel Brakel Breda Breda Breda Bresse-Gauloise Bresse-Gauloise Bresse-Gauloise Brügger Kämpfer Combattant de Bruges Bruges game Campiner Campine Campine Cemani Cemani Cemani Cochin Cochin Cochin Crèvecoeur Crèvecoeur Crèvecoeur Croad Langschan Croad Langshan Croad Langshan Cubalaya Cubalaya Cubalaya Dänische Landhühner Poule danoise Danish fowl Dänische Lutterhühner Lutter danaoise Danish Lutter Deutsche Faverolles Faverolles allemande German Faverolles Deutsche Langschan Langshan allemande German Langshan Deutsche Reichshühner Poule du Reich German Reichshühner Deutsche Sperber Coucou allemand German cuckoo Deutsche Wyandotten Wyandotte allemande German Wyandotte Drenther Hühner -

Genetic Variation in the Chicken Genome: Insights in Selection
Genetic variation in the chicken genome: insights in selection Martin Elferink Thesis committee Thesis supervisor Prof. dr. M.A.M. Groenen Personal chair at Animal Breeding and Genomics Centre Wageningen University Thesis co-supervisor Dr. R.P.M.A. Crooijmans Assistant Professor at Animal Breeding and Genomics Centre Wageningen University Other Members Prof. dr. ir. F.P.M. Govers Wageningen University Prof. dr. J.H.S.G.M. de Jong Wageningen University Prof. dr. M. Pérez-Enciso Universitat Autònoma de Barcelona, Bellaterra, Spain Dr. M. Tixier-Boichard Ministère de l'Enseignement supérieur et de la Recherche, Paris, France This research was conducted under the auspices of the Wageningen Institute of Animal Sciences (WIAS) graduate school Genetic variation in the chicken genome: insights in selection Martin Elferink Thesis submitted in fulfilment of the requirements for the degree of doctor at Wageningen University by the authority of the Rector Magnificus Prof. dr. M.J. Kropff, in the presence of the Thesis Committee appointed by the Academic Board to be defended in public on Monday 20 June 2011 at 4 p.m. in the Aula. Martin Gerhard Elferink Genetic variation in the chicken genome: insights in selection, 164 pages Thesis, Wageningen University, Wageningen, NL (2011) With references, with summaries in Dutch and English ISBN 978-90-8585-920-8 Contents Chapter 1 General introduction 7 Chapter 2 Regional differences in recombination hotspots 35 between two chicken populations Chapter 3 Partial duplication of the PRLR and SPEF2 genes at 55 the late feathering locus in chicken Chapter 4 Massive parallel sequencing of 12 genomes identifies 73 protein affecting variants within QTL regions associated with the pulmonary hypertension syndrome in chicken.