Genetic Analysis of Production Traits to Support Breeding Programs Utilizing Indigenous Chickens in Ethiopia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FAMILY POULTRY DEVELOPMENT Working Paper Issues, Opportunitiesandconstraints FAO ANIMALPRODUCTIONANDHEALTH 12
12 ISSN 2221-8793 FAO ANIMAL PRODUCTION AND HEALTH working paper FAMILY POULTRY DEVELOPMENT Issues, opportunities and constraints Cover photographs Left image: ©Brigitte Bagnol Centre image: ©IFAD/Antonio Rota Right image: ©FAO/Olaf Thieme 12 FAO ANIMAL PRODUCTION AND HEALTH working paper FAMILY POULTRY DEVELOPMENT Issues, opportunities and constraints Authors Olaf Thieme, Emmanuel Babafunso Sonaiya, Antonio Rota, Robyn G. Alders, Mohammad Abdul Saleque and Giacomo De’ Besi FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 2014 Recommended Citation FAO. 2014. Family poultry development − Issues, opportunities and constraints. Animal Production and Health Working Paper. No. 12. Rome. Authors Olaf Thieme Livestock Production Systems Branch, Animal Production and Health Division, FAO, Rome, Italy [email protected] Emmanuel Babafunso Sonaiya Department of Animal Sciences, Obafemi Awolowo University, Ile-Ife, Nigeria [email protected] Antonio Rota Policy and Technical Advisory Division (PTA), IFAD, Rome, Italy [email protected] Robyn G. Alders International Rural Poultry Centre (IRPC) of the KYEEMA Foundation, Australia; and Charles Perkins Centre and Faculty of Veterinary Science, University of Sydney, Australia [email protected] [email protected] Mohammad Abdul Saleque BRAC International, BRAC Centre, Dhaka, Bangladesh [email protected] Giacomo De’ Besi Private consultant [email protected] The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

Poultry As a Development Tool
PART 3 Poultry as a Development Tool 503 Poultry production for livelihood improvement and poverty alleviation Frands Dolberg University of Århus, Denmark SUMMARY Millennium Development Goal Number One is to halve the number of poor people in the world by 2015. The present paper contains a discussion, based on the livelihoods frame- work, of how and under what conditions small poultry units can contribute to the achieve- ment of this and other Millennium Development Goals. The paper presents the livelihoods framework along with its micro- and macro-level features. Subsequently, it discusses the role of poultry in asset creation and as an entry point to improved livelihoods. A series of cases are presented: from Afghanistan, Bangladesh, Egypt, the Lao People’s Democratic Republic and Swaziland, which illustrate various arguments related to the use of poultry for livelihood improvement and poverty alleviation. Strategies that use poultry production for livelihood improvement and poverty alleviation will be most relevantly applied in the countries where it has been most difficult to get development moving. These countries are variously described by development agencies as low-income countries under stress (LICUS), highly indebted poor countries (HIPC), low-income food deficit countries (LIFDCs), or countries that are placed low on the UN Human Development Index. The smallholder poultry approach is biased towards poor women; one estimate is that it is relevant for 160 million women and their families. However, it will not be easy to reach these potential beneficiaries, as bad governance and weak institutions characterize many of the countries where they live. Against this background, it is concluded that international organizations and networks have a particularly important role to play as storehouses of knowledge and technical expertise. -

Efficacy of in Ovo Delivered Prebiotics on Growth Performance, Meat Quality and Gut Health of Kuroiler Chickens in the Face of A
animals Article Efficacy of In Ovo Delivered Prebiotics on Growth Performance, Meat Quality and Gut Health of Kuroiler Chickens in the Face of a Natural Coccidiosis Challenge Harriet Angwech 1,2,*, Siria Tavaniello 1, Acaye Ongwech 1,2, Archileo N. Kaaya 3 and Giuseppe Maiorano 1 1 Department of Agricultural, Environmental and Food Sciences, University of Molise, 86100 Campobasso, Italy; [email protected] (S.T.); [email protected] (A.O.); [email protected] (G.M.) 2 Department of Biology, Faculty of Science, Gulu University, P.O. Box 166 Gulu, Uganda 3 School of Food Technology, Nutrition and Bio-engineering, Makerere University, P.O. Box 7062 Kampala, Uganda; [email protected] * Correspondence: [email protected] Received: 6 August 2019; Accepted: 29 September 2019; Published: 28 October 2019 Simple Summary: The management of coccidiosis in poultry farms is mainly dependent on the use of anticoccidial drugs. Development of resistance to existing anticoccidial drugs coupled with restrictive use of antibiotics to control secondary bacterial infections following the ban on antibiotics, stresses the urgent need to explore alternative strategies for maintaining intestinal functionality in chickens for improved productivity. Prebiotics have been proposed as a solution to the intestinal problems of poultry. This study demonstrates that in ovo delivered prebiotics with or without antibiotics reduces severity of intestinal lesions and oocyst excretion induced by natural infection with Eimeria. Prebiotics protected Kuroiler chickens from coccidia in particular in the first 56 days of age and tended to have a synergistic effect with anticoccidial drug in the management of the disease post-infection in the field, with positive effects on performance and meat quality. -

211.Full.Pdf
MOLECULAR GENETICS OF AVIAN PROTEINS. IX. INTERSPECIFIC AND INTRASPECIFIC VARIATION OF EGG WHITE PROTEINS OF THE GENUS GALLUS C. M. ANN BAKER 2, Bridge End, Berwick-upon-Tweed, England Received July 11, 1967 egg white of the domestic fowl, Gallus gallus L., consists of at least ten T?ff erent proteins: ovalbumin, conalbumin, ovomucoid, ovoinhibitor, mucin, flavoprotein. avidin, and globulins G, (= lysozyme), G, and G,. In starch gel electrophoresis many of these are heterogeneous and four have genetically con- trolled polymorphisms, which are described below. (The electrophoretic patterns of many of the variants are in Figure 1.) Ovalbumin (ou locus). Alleles ou A and ou B result in phenotypes A, B, and AB (LUSH1961, 1964b). In the present paper, however, the variants are named with reference to their relative mobilities as the letter A is used in A,, A, and A, terminology for the sub-fractions of ovalbumin ( LONGSWORTH,CANNAN and MAC- INNES1940; CANN1949). Thus ovalbumin A = slow (S) and ovalbumin B = fast (F). A recent preliminary communication suggests that the primary struc- ture of the fast and slow ovalbumins differs in a single peptide (WISEMANand FOTHERGILL1966). Other ovalbumin variants have been described. BAKERand MANWELL(1 962) found polymorphism which involves a more rapid migration of A, and A, and a lack of A, in one homozygote category (A3--) ; the heterozygote (A?+-) has less of the A, subfraction than the “normal” homozygote (A,++). CROIZIER(1966) has discovered yet another variant (ovalbumin Faverolles) which has subfrac- tions extra to those of the fast and slow variants. He also found it possible to clas- sify each of LUSH’S(1964b) ovalbumin phenotypes into two subtypes on the basis of quantitative differences in A,. -

Copyrighted Material
FTOC 08/27/2018 10:24:30 Page v Contents Acknowledgements ix Introduction 1 Standard feather markings 4 Chief points of the fowl 13 Complete classification of pure breed poultry 21 Defects and deformities 25 Large fowl and bantams 31 Ancona 31 Andalusian 34 Appenzeller 36 Araucana 41 Rumpless Araucana 45 Asil 48 Australorp 50 Autosexing breeds 53 Brockbar 54 Brussbar 55 Cambar 57 Dorbar 59 Legbar 60 Rhodebar 63 Welbar 65 Wybar 68 Ayam Cemani 71 Barnevelder 72 Belgian Bearded bantamsCOPYRIGHTED MATERIAL 75 Barbu d’Anvers 75 Barbu d’Uccle 77 Barbu de Watermael 78 Barbu d’Everberg (Rumpless d’Uccle) 80 Barbu du Grubbe (Rumpless d’Anvers) 81 Barbu de Boitsfort (Rumpless de Watermael) 81 Booted bantam 87 Rumpless Booted Bantam 92 Brabanter 93 Brahma 95 Brakel 100 FTOC 08/27/2018 10:24:30 Page vi vi Contents Breda 102 Bresse-Gauloise 104 Burmese 106 Campine 108 Cochin 111 Crèvecoeur 114 Croad Langshan 115 Dandarawi 118 Derbyshire Redcap 120 Dominique 121 Dorking 124 Dutch bantam 128 British Faverolles 133 Fayoumi 137 Friesian 139 Frizzle 143 German Langshan 145 Groninger 149 Hamburgh 152 Houdan 155 Indian Game 158 Ixworth 162 Japanese bantam 164 Jersey Giant 169 Ko Shamo 171 Kraienköppe 173 Kulang 177 La Flèche 179 Lakenvelder 181 Leghorn 183 Lincolnshire Buff 188 Malay 191 Marans 194 Marsh Daisy 198 Minorca 201 Modern Game 204 Modern Langshan 209 Nankin bantam 212 Nankin Shamo 214 New Hampshire Red 215 Norfolk Grey 218 North Holland Blue 220 Ohiki 222 Carlisle Old English Game 223 Oxford Old English Game 230 Old English Game bantam 236 Old English -

Poultry in the United Kingdom the Genetic Resources of the National Flocks
www.defra.gov.uk Poultry in the United Kingdom The Genetic Resources of the National Flocks November 2010 Cover: Red Dorking male (photograph John Ballard, courtesy of The Cobthorn Trust) All information contained in this brochure was correct at time of going to press (December 2010). Department for Environment, Food and Rural Affairs Nobel House 17 Smith Square London SW1P 3JR Telephone: 020 7238 6000 Website: www.defra.gov.uk © Crown copyright 2010 Copyright in the typographical arrangement and design rests with the Crown. This publication (excluding the logo) may be reproduced free of charge in any format or medium provided that it is reproduced accurately and not used in a misleading context. The material must be acknowledged as Crown copyright with the title and source of the publication specified. This document is also available on the Defra website. Published by the Department for Environment, Food and Rural Affairs. Printed in the UK, December 2010, on material that contains a minimum of 100% recycled fibre for uncoated paper and 75% recycled fibre for coated paper. PB13451 December 2010. Contents 1. Introduction 3 2. Poultry keeping systems 4 3. Species Accounts 6 3.1. The Domestic Fowl (Gallus gallus domesticus) 6 3.2. Turkeys 8 3.3. Ducks 8 3.4. Geese 9 3.5. Minor Species 9 4. Breed Organisations 10 5. Data Recording and Registration 11 6. References 12 7. Annex: Current situation for individual breeds and strains 13 Abbreviations 24 1 1. Introduction Domestic poultry form the most important sector of livestock keeping worldwide, the production of meat and eggs being a major contributor to human nutrition. -

Decision Tools for Family Poultry Development Rural, Urban and Peri-Urban Areas of Developing Countries
16 16 ISSN 1810-0708 FAO ANIMAL PRODUCTION AND HEALTH Family poultry encompasses all small-scale poultry production systems found in Decision tools for family poultry development rural, urban and peri-urban areas of developing countries. Rather than defining the production systems per se, the term is used to describe poultry production practised by individual families as a means of obtaining food security, income and gainful employment. Family poultry production is often perceived as an activity that can easily and quickly generate income and support food security for resource-poor households. However, the essential requirements for the efficient production of healthy and profitable poultry and eggs are frequently inadequately understood by those designing projects for resource-poor settings. This publication provides guidance for personnel in governments, development organizations and NGOs to better determine and plan development interventions for family poultry. guidelines The decision tools address the situation of four distinct family poultry production systems and their development opportunities: small extensive scavenging, extensive scavenging, semi-intensive production and small-scale intensive production. They describe the poultry production systems, including their required inputs and expected outputs and the techniques and tools used to assess the operational environment, in order to design interventions suited to the local conditions. Practical technical information are provided about genetics and reproduction, feeds and feeding, -

British Poultry Standards
British Poultry Standards Complete specifi cations and judging points of all standardized breeds and varieties of poultry as compiled by the specialist Breed Clubs and recognised by the Poultry Club of Great Britain Sixth Edition Edited by Victoria Roberts BVSc MRCVS Honorary Veterinary Surgeon to the Poultry Club of Great Britain Council Member, Poultry Club of Great Britain This edition fi rst published 2008 © 2008 Poultry Club of Great Britain Blackwell Publishing was acquired by John Wiley & Sons in February 2007. Blackwell’s publishing programme has been merged with Wiley’s global Scientifi c, Technical, and Medical business to form Wiley-Blackwell. Registered offi ce John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, United Kingdom Editorial offi ce 9600 Garsington Road, Oxford, OX4 2DQ, United Kingdom For details of our global editorial offi ces, for customer services and for information about how to apply for permission to reuse the copyright material in this book please see our website at www.wiley.com/wiley-blackwell. The right of the author to be identifi ed as the author of this work has been asserted in accordance with the Copyright, Designs and Patents Act 1988. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher. Wiley also publishes its books in a variety of electronic formats. Some content that appears in print may not be available in electronic books. -
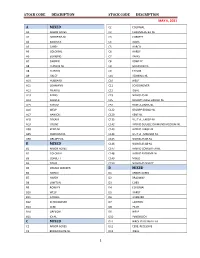
Stock Codes Numerical
STOCK CODE DESCRIPTION STOCK CODE DESCRIPTION MAY 6, 2021 A MIXED C2 COLONIAL A1 ARBOR ACRES C3 CHAUMIERE BB-NL A2 ANDREWS-NL C3 CORBETT A2 BABCOCK C4 DAVIS A3 CAREY C5 HARCO A5 COLONIAL C6 HARDY A6 EURIBRID C7 PARKS A7 GARBER C8 ROWLEY A8 H AND N-NL C9 GUILFORD-NL A8 H AND N C9 TATUM A9 HALEY C10 HENNING-NL A10 HUBBARD C10 WELP A11 LOHMANN C11 SCHOONOVER A12 MERRILL C12 IDEAL A13 PARKS C19 NICHOLAS-NL A14 SHAVER C35 ORLOPP LARGE BROAD-NL A15 TATUM C57 ROSE-A-LINDA-NL A16 WELP C122 ORLOPP BROAD-NL A17 HANSON C129 KENT-NL A18 DEKALB C135 B.U.T.A., LARGE-NL A19 HYLINE C142 HYBRID DOUBLE DIAMOND MEDIUM-NL A38 KENT-NL C143 HYBRID LARGE-NL A45 MARCUM-NL C144 B.U.T.A., MEDIUM-NL A58 ORLOPP-NL C145 NICHOLAS 85-NL B MIXED C146 NICHOLAS 88-NL B1 ARBOR ACRES C147 HYBRID CONVERTER-NL B2 COLONIAL C148 HYBRID EXTREME-NL B3 CORBETT C149 MIXED B4 DAVIS C150 NICHOLAS SELECT B5 DEKALB WARREN D MIXED B6 HARCO D1 ARBOR ACRES B7 HARDY D2 BRADWAY B8 LAWTON D3 COBB B9 ROWLEY D4 COLONIAL B10 WELP D5 HARDY B11 CARGILL D6 HUBBARD B12 SCHOONOVER D7 LAWTON B13 CEBE D8 PILCH B14 OREGON D9 WELP B15 IDEAL D10 PENOBSCOT C MIXED D11 WROLSTAD SMALL-NL C1 ARBOR ACRES D11 CEBE, RECESSIVE C2 BROADWHITE-NL D12 IDEAL 1 STOCK CODE DESCRIPTION STOCK CODE DESCRIPTION MAY 6, 2021 N14 OLD ENGLISH, WHITE E MIXED N15 OLD ENGLISH, BLACK E1 COLONIAL N16 OLD ENGLISH, SPANGLED E2 HUBBARD N17 PIT E3 BOURBON, RED-NL N18 OLD ENGLISH E3 ROWLEY N19 MODERN E4 WELP N20 PIT, WHITE HACKLE E5 SCHOONOVER N21 SAM BIGHAM E6 CEBE N22 MCCLANHANS H MIXED N23 CLIPPERS H1 ARBOR ACRES N24 MINER BLUES H2 -

Epidemiology of Important Poultry Diseases in Nepal
Nepalese Vet. J. 36: 08 –14 Epidemiology of Important Poultry Diseases in Nepal T. R. Gompo*,1, U. Pokhrel2, B. R. Shah3,D. D. Bhatta1 1 Department of Livestock Services, Central Veterinary Laboratory, Kathmandu, Nepal 2 Agriculture and Forestry University, Chitwan, Nepal 3Institute of Agriculture and Animal Science, Tribhuvan University *Corresponding author: [email protected] ASBTRACT Despite the rapidly growing poultry industry throughout Nepal, the periodic outbreaks of diseases and infections in poultry birds led to huge production loss. The aim of this study was to identify the top ten poultry diseases in Nepal and an analysis of their seasonal distributions. A cross-sectional study was performed to describe the distributions of major poultry diseases diagnosed from April 2018 to April 2019 at Central Veterinary Laboratory, Nepal. Out of 2358 observations recorded at the CVL registry at that period, only 2271 observations qualified for the final analysis. Among 2271, removing the missing values, only 1915 observations were used to describe bird characteristics such as median age and mean flock sizes. Descriptive analysis and graphical representation was performed in R studio (Version 1.0.143) and MS excel 2010 respectively. The top ten diseases identified with highest to lowest incidence were: colibacillosis 26% (584/2271), mycotoxicosis 13% (301/2271), ascites 10% (232/2271), complicated chronic respiratory disease (cCRD) 9% (196/2271), infectious bursal disease (IBD) 7% (155/2271), Newcastle disease (ND) 7% (148/2271), avian influenza (AI) 3% (76/2271), salmonellosis 2% (40/2271), infectious bronchitis 1% (33/2271), coccidiosis 1% (25/2271) and non-specific diseases accounts for 21% (481/2271). -

RARE POULTRY CLASSES 8 Malay Pullet
ASIAN HARDFEATHER 36 Ko Shamo Black/White/Self Pullet Regional Show 37 Ko Shamo AOC Male Sec: Z Shakeshaft Tel: 07866 575316 38 Ko Shamo AOC Female Judges : Mr R Francis (Large Fowl) 39 Ko Shamo AOC Stag & Mr P Cox ( Bantams) 40 Ko Shamo AOC Pullet 0 41 Asil Male LARGE FOWL 42 Asil Female 43 Tuzo Male 1 Asil Male 44 Tuzo Female 2 Asil Female 45 Non Standard/AOV Male 3 Asil Stag 46 Non Standard/AOV Female 4 Asil Pullet See Juvenile section for entries 5 Malay Male 6 Malay Female 7 Malay Stag RARE POULTRY CLASSES 8 Malay Pullet 9 O Shamo Male (8.8lbs+) RARE POULTRY SOCIETY 10 O Shamo Female (6.6lbs+) Club Show 11 Chu Shamo Male (Under 8.8lbs) Sec: P M Fieldhouse Tel: 01934 12 Chu Shamo Female (under 6.6lbs) 824213 (eve) 13 Shamo Stag (Any size) Judges: Mr J Lockwood 14 Shamo Pullet (Any Size) (Hardfeather & Soft Feather Heavy) 15 Kulang Male Mr P Hayford (True Bantams, 16 Kulang Female Longtails, Juveniles, Pairs & Trios) 17 Satsumadori Male Mr A Hillary (Soft Feather Light 18 Satsumadori Female Breeds 1) 19 Yamato Gunkei Male Mr J Robertson (Soft Feather Light 20 Yamato Gunkei Female Breeds 2) 21 Yamato Gunkei Stag 22 Yamato Gunkei Pullet LONG TAILED LARGE 23 Non Standard/AOV Male 24 Non Standard/AOV Female 47 Yokohama Male See Juvenile section for entries 48 Sumatra Male 49 Yokohama Female BANTAMS 50 Sumatra Female 25 Malay Male LARGE SOFTFEATHER LIGHT 26 Malay Female BREEDS 27 Ko Shamo Black Red Male 28 Ko Shamo Black Red Stag 51 Andalusian Male 29 Ko Shamo Duckwing Male 52 Andalusian Female 30 Ko Shamo Duckwing Stag 53 Ayam Cemani M/F -

Comparison of Production Parameters and Meat Quality Characteristics of South African Indigenous Chickens
Comparison of Production Parameters and Meat Quality Characteristics of South African Indigenous Chickens By Russel Packard Thesis presented in partial fulfilment of the requirements for the degree of Master of Science in Agriculture at Stellenbosch University Faculty of AgriSciences Department of Animal Sciences Supervisor: Dr. Elsje Pieterse Co-Supervisor: Prof. Louw Hoffman Date: April 2014 Stellenbosch University http://scholar.sun.ac.za Declaration By submitting this thesis electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated), that reproduction and publication thereof by Stellenbosch University will not infringe on third party rights and that I have not previously in its entirety or in part submitted it for obtaining any qualification. Date: April 2014 Copyright © 2014 stellenbosch University All rights reserved ii Stellenbosch University http://scholar.sun.ac.za Abstract This study quantified the growth performance, carcass and meat characteristics of South African slow-growing chicken lines. Two slow-growing lines developed outside South Africa, the Black Australorp and New-Hampshire, two native lines including the Naked-Neck and Potchefstroom Koekoek, as well as a hybrid between a Cobb 500 broiler and Potchefstroom Koekoek were evaluated. Fifty birds of each line were randomly allocated to cages of five birds per cage where they were fed a standard broiler diet ad libitum to an average weight of 2kg. Twenty cockerels of each line were then slaughtered for further analyses. For the carcass characteristics: live weight at slaughter, hot carcass weight, and chilled carcass weight were determined.