Microscopic Colitis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Microscopic Colitis: Collagenous Colitis and Lymphocytic Colitis
Microscopic Colitis: Collagenous Colitis and Lymphocytic Colitis National Digestive Diseases Information Clearinghouse What is microscopic colitis? The term bowel refers to any part of the small or large intestine. The large intes- Microscopic colitis is inflammation of the tine includes the colon and the rectum, and bowel that is only visible using a microscope. together they are about 5 feet long. The Microscopic colitis is a type of inflammatory U.S. Department small intestine can be 12 to 20 feet long. bowel disease (IBD), which refers to a group of Health and Colitis means inflammation of the colon. Human Services of conditions that causes inflammation in the Microscopic colitis is inflammation of the bowel due to an excessive build-up of white colon and rectum. NATIONAL blood cells in the bowel lining. Microscopic INSTITUTES OF HEALTH colitis is less severe than other types of IBD because it does not lead to cancer and rarely What are collagenous colitis requires surgery. However, microscopic and lymphocytic colitis? colitis can cause considerable pain and Microscopic colitis has two main forms: discomfort. collagenous colitis and lymphocytic colitis. The symptoms of and treatment for both are identical. Some scientists believe the two forms may be different presentations of the same disease. Slight differences in the way intestinal tissues appear when seen with a microscope set them apart. In both forms, an increase in white blood cells can be seen within the intestinal epithelium—the layer of Stomach Liver cells that lines the intestine. Increased white blood cells are a sign of inflammation. But with collagenous colitis, the layer of collagen Colon (shaded) beneath the epithelium appears thicker than normal. -

Associated Ulcerative Colitis, Sclerosing Cholangitis, and Insulin*Dependent Diabetes Mellitus
CASE REPORT Associated ulcerative colitis, sclerosing cholangitis, and insulin*dependent diabetes mellitus MARSHA KAY, MD; ROBERT WYLLIE, MD; WILLIAM MICHENER, MD; MAUREEN CAULFIELD, MD; RITA STEFFEN, MD LINICALLY symptomatic We report two young men with clinical and laboratory evidence ulcerative colitis, sclero- of macroscopic ulcerative colitis, sclerosing cholangitis, and insu- sing cholangitis, and in- lin-dependent diabetes mellitus. The first patient presented at age Csulin-dependent diabe- 15 with vomiting, abdominal pain, weight loss, and abnormal tes mellitus have not previously liver function test results. Liver biopsy and endoscopic retrograde been reported in the same patient. cholangiopancreatography (ERCP) demonstrated sclerosing cho- Although each may be associated langitis. Colonoscopy with biopsy revealed ulcerative colitis with the other, their occurrence in which responded to sulfasalazine. Diabetes occurred at age 18 and the same individual implies a com- insulin therapy was begun. mon susceptibility, perhaps involv- The second patient was 19 at presentation with diarrhea, hema- ing the immune system. We have tochezia, and weight loss. Proctosigmoidoscopy revealed identified two patients with each ulcerative colitis, and sulfasalazine led to clinical remission. Three of these disorders. These two months later he developed diabetes requiring insulin therapy. At young men were followed up at age 28, he developed elevated alkaline phosphatase, and ERCP re- The Cleveland Clinic Foundation vealed sclerosing cholangitis. At age 37 he expired from adenocar- between 1970 and 1992. cinoma that metastasized to the liver. Literature review revealed only one possible case report of this PATIENT I; CASE HISTORY association with microscopic asymptomatic ulcerative colitis in that patient. Statistical analysis suggests that this association is Patient 1 presented at age 15 real rather than a chance occurrence. -

Clinical and Pathological Aspects of Inflammatory Bowel Disease
Inflammatory Bowel Diseases: B.R. Bistrian; J.A. Walker-Smith (eds), Nestlé Nutrition Workshop Series Clinical & Performance Programme, Vol. 2, pp. 83–92, Nestec Ltd.; Vevey/S. Karger AG, Basel, © 1999. Clinical and Pathological Aspects of Inflammatory Bowel Disease Ph. Marteau Gastroenterology Department, European Hospital Georges Pompidou, Paris, France The term “inflammatory bowel disease” applies to bowel diseases of unknown etiology characterized by chronic and often relapsing inflammation. They include ulcerative colitis, Crohn’s disease, indeterminate colitis, pouchitis, and micro- scopic colitides. Although these diseases share a number of epidemiological, pathological, and clinical features, they differ sufficiently to be classified as dis- tinct entities. The term “indeterminate colitis” is used for colitides which do not present enough criteria to be classified as ulcerative colitis or Crohn’s disease. Ulcerative Colitis Pathology Ulcerative colitis is a mucosal disease, which always affects the rectum and often also involves a variable contiguous proximal segment of colonic mucosa [1]. The lesions are continuous, and their upper limit is sharply demarcated from the normal mucosa above. They are limited to the rectum in about 25% of the patients (proctitis); reach the sigmoid colon in another 25% (proctosigmoiditis); spread to the splenic flexure in another 25% (left-sided colitis), and affect the whole colon in about 15% (pancolitis). The small intestine is usually normal but may be occasionally involved by superficial inflammation (“backwash ileitis”) in some patients with pancolitis. Macroscopic lesions can be evaluated during endoscopic examination [2]. Active lesions consist of edema, erythema, lack of the normal vascular pattern, bleeding, exudation of mucus or pus, and ulceration (Table 1). -

Microscopic Colitis: Collagenous Colitis and Lymphocytic Colitis
A SPECIAL ARTICLE Microscopic Colitis: Collagenous Colitis and Lymphocytic Colitis Brennan A. Scott Thomas P. Prindiville Collagenous colitis and lymphocytic colitis are chronic relapsing diarrheal illnesses, which are often referred to together as microscopic colitis. It most commonly occurs in women in their fifth to sixth decade. The symptoms usually include profuse watery diarrhea and crampy abdominal pain. Laboratory and endoscopic studies are gener- ally normal but microscopic inflammation is seen when colonic biopsies are performed. In collagenous colitis there is a subepithelial collagen band in addition to chronic inflammation in the lamina propria. The etiology is not known but multiple theories exist including autoimmune, infectious, and medication-induced. Although the course is generally benign, patients may have multiple relapses over many years. Treatment regimens vary and have included anti-diarrheals, antibiotics, 5-aminosalicylates, steroids, and immunosuppressive agents. INTRODUCTION abdominal pain. Both conditions have normal mucosa ollagenous colitis (CC) (Figure 1) and lympho- when viewed endoscopically, however biopsy speci- cytic colitis (LC) (Figure 2) are uncommon mens show chronic mucosal inflammation. In CC C chronic relapsing diarrheal illnesses. The major- there is a subepithelial collagen band of varying thick- ity of patients are women in their fifth to sixth decade ness in association with an inflammatory cell infiltrate who complain of profuse, watery diarrhea, and crampy in the lamina propria (1). The collagen band is absent in LC (2,3). The term microscopic colitis (MC) was Brennan A. Scott, MD, Clinical Fellow, Division of Gas- originally used to describe patients with chronic diar- troenterology, University of California, Davis Medical rhea and normal endoscopic and barium enema stud- Center. -
Microscopic Colitis Collagenous and Lymphocytic Colitis Publisher
The informed patient Microscopic colitis Collagenous and lymphocytic colitis Publisher © 2020 Dr. Falk Pharma GmbH 6th updated and All rights reserved. revised edition 2020 The informed patient Microscopic colitis Collagenous and lymphocytic colitis Prof. Dr. Andreas Tromm, Hattingen (Germany) Microscopic colitis – Collagenous and lymphocytic colitis Address of the author Prof. Dr. Andreas Tromm Klinik für Innere Medizin Augusta-Kranken-Anstalt gGmbH Betriebsstelle EVK Hattingen Akademisches Lehrkrankenhaus der Universität Duisburg-Essen Bredenscheider Str. 54 45525 Hattingen Germany www.klinik-gastroenterologie.de Contents Introduction 4 Clinical presentation 6 Causes and development of microscopic colitis 9 Diagnosis 11 Treatment 14 Frequently asked questions about microscopic colitis 17 3 Microscopic colitis – Collagenous and lymphocytic colitis Introduction The term microscopic colitis encompasses two different disorders of the colon known as collagenous colitis and lymphocytic colitis. Both disorders are characterized by non-bloody watery diarrhea and are sometimes referred to as watery diar- rhea syndromes (Fig. 1). Microscopic colitis Collagenous colitis Lymphocytic colitis Watery diarrhea syndrome Fig. 1: Definition of microscopic colitis. 4 Introduction The term microscopic colitis describes a chronic in- flammatory disease of the colon (“colitis” comes from the Latin term “colon” and the ending “-itis”, which is used in medicine to refer to inflammation) that a doctor cannot identify by colonoscopy with the naked eye because the mucosa of the colon appears normal. In order for the disease to be diagnosed, the doctor must therefore remove a small tissue sample and examine it under a microscope. This is the only way to diagnose microscopic colitis. For collagenous colitis, a thickened collagen layer becomes visible when the tissue samples are stained using special methods, whereas lymphocytic colitis is detected as an increased number of a specific type of white blood cells called lymphocytes (see page 12). -

Microscopic Colitis What Is Microscopic Colitis?
Microscopic Colitis What is microscopic colitis? Microscopic colitis is an inflammation of the colon that a health care provider can see only with a microscope. Inflammation is the body’s normal response to injury, irritation, or infection of tissues. Microscopic colitis is a type of inflammatory bowel disease—the general name for diseases that cause irritation and inflammation in the intestines. The two types of microscopic colitis are collagenous colitis and lymphocytic colitis. Health care providers often use the term microscopic colitis to describe both types because their symptoms and treatments are the same. Some scientists believe that collagenous colitis and lymphocytic colitis may be different phases of the same condition rather than separate conditions. In both types of microscopic colitis, an increase in the number of lymphocytes, a type of white blood cell, can be seen in the epithelium—the layer of cells that lines the colon. An increase in the number of white blood cells is a sign of inflammation. The two types of colitis affect the colon tissue in slightly different ways: Lymphocytic colitis. The number of lymphocytes is higher, and the tissues and lining of the colon are of normal thickness. Collagenous colitis. The layer of collagen, a threadlike protein, underneath the epithelium builds up and becomes thicker than normal. When looking through a microscope, the health care provider may find variations in lymphocyte numbers and collagen thickness in different parts of the colon. These variations may indicate an overlap of the two types of microscopic colitis. What causes microscopic colitis? The exact cause of microscopic colitis is unknown. -

Lymphocytic Colitis: a Retrospective Clinical Study of 199 Gut: First Published As 10.1136/Gut.2003.023440 on 11 March 2004
536 INFLAMMATION Lymphocytic colitis: a retrospective clinical study of 199 Gut: first published as 10.1136/gut.2003.023440 on 11 March 2004. Downloaded from Swedish patients M Olesen, S Eriksson, J Bohr, G Ja¨rnerot, C Tysk ............................................................................................................................... Gut 2004;53:536–541. doi: 10.1136/gut.2003.023440 Background: Lymphocytic colitis is characterised by chronic diarrhoea and specific microscopic changes in a macroscopically normal colonic mucosa. We report clinical features and treatment outcome in a large patient cohort. Methods: Patients were searched for in 24 Swedish gastroenterology clinics. The biopsy material was reassessed using strict histopathological criteria. Clinical data were obtained from medical notes. See end of article for Results: Lymphocytic colitis was diagnosed in 199 cases. The female:male ratio was 2.4:1. Median age at authors’ affiliations diagnosis was 59 (48–70) years. The most frequent symptoms were diarrhoea (96%), abdominal pain ....................... (47%), and weight loss (41%). The course was chronic intermittent in 30% of patients, chronic continuous in Correspondence to: 7%, and a single attack in 63%, and in these cases the disease duration was 6 (4–11) months. Seventy Associate Professor nine (40%) patients reported associated diseases, of which thyroid disorders, coeliac disease, and C Tysk, Department of diabetes mellitus were the most common. In 34 first or second degree relatives of 24 (12%) patients, a Medicine, Division of family history of ulcerative colitis, Crohn’s disease, collagenous colitis, or coeliac disease was reported. Gastroenterology, O¨ rebro University Hospital, Drug induced disease was suspected in 19 (10%) patients. A non-significant peak of disease onset was 701 85 O¨ rebro, Sweden; seen in December-January. -

What Is Colitis? Pitfalls in the Microscopic Diagnosis
What is colitis? Pitfalls in the microscopic diagnosis K. Geboes, KULeuven, Bucarest 2011 What is colitis? Statistical approach (morphometry)? • Chronic inflammatory infiltration total cellularity increase • Surface epithelial height to crypt epithelial height. In normal mucosa the surface epithelial cell height exceeds the height of crypt epithelium • Redistribution of infiltrating cells so that there is a similar density in the basal third to that of the superficial third > IBD Jenkins e.a. J Clin Pathol 1988; 41; 72-79 1 Normal mucosa vs Colitis – Lamina propria cellular infiltrate : increase in intensity; composition & distribution – Organized lymphoid tissue : increase, stimulation – Epithelial cells : • surface epithelium – terminally differentiated cells DAMAGE & REPAIR (restitution) • crypts – differentiating cells, proliferative compartment INCREASED PROLIFERATION (mitotic activity) • normal turnover : increased turnover Basic lesions : Inflammation • Inflammation pattern I – Patchy, focal – Diffuse 2 Basic lesions : Inflammation • Inflammation pattern II – Diffuse upper third (Infections such as Shigella colitis) – Diffuse transmucosal (IBD) Basic lesions : Architecture • Surface – Flat or irregular 3 Basic lesions : Architecture • Crypt architecture – Crypt density • 7/8 crypts per 1 mm mucosal length (IBD 4 to 5) • Closely packed – Variable or constant intercryptal distance Basic lesions : Architecture • Crypt architecture – Straight or branching tubes (infrequent branching < 10% may be normal) – Base reaching muscularis mucosae -

Advances in Digestive Diseases 2019: Highlights from DDW 6/1/19 Richard Kalman, MD Director Liver Tumor Program [email protected] Outline
Recent Advances in Autoimmune Gastrointestinal Disorders Advances in Digestive Diseases 2019: Highlights from DDW 6/1/19 Richard Kalman, MD Director Liver Tumor Program [email protected] Outline • Celiac Disease • Microscopic Colitis • Autoimmune Hepatitis • IBD • EoE • Autoimmune gastritis • Autoimmune enteropathy • PBC • PSC Richard Kalman [email protected] Outline • Celiac Disease • Microscopic Colitis • Autoimmune Hepatitis • IBD • EoE • Autoimmune gastritis • Autoimmune enteropathy • PBC • PSC Richard Kalman [email protected] Celiac Disease Richard Kalman [email protected] Celiac Disease • Immune mediated small intestine enteropathy • Partial gluten digestion • Bind HLA DQ on APCs – Activate CD4+ T cells • Proinflammatory cytokine • Stimulates B cell response – Promote IEL activation • Transform to NK like cells • Destroy enterocytes Tye-Din JA et al. Front Pediat. 2018 Celiac Disease • Injury → less absorptive area → reduction in digestive enzymes → micronutrient deficiencies – fat soluble vitamins, iron, B12, Folic acid • Inflammation exacerbates malabsorption by causing secretion of fluid that increases diarrhea • 100% of patients possess specific variants of HLA class II genes - HLA-DQ2 and DQ8 – 90% DQ2 – 20-30% of US population carry these genes– only 2- 3% of these people will get CD Lebwohol B et al. Lancet. 2018 Celiac Disease • Presentation – Asymptomatic to severe malnutrition – GI – pain, bloating, diarrhea, dyspepsia, weight loss, steatorrhea – Extra-intestinal – fatigue, joint pain, cognitive -
Microscopic Colitis (PDF)
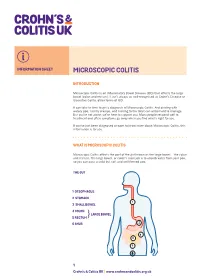
INFORMATION SHEET MICROSCOPIC COLITIS INTRODUCTION Microscopic Colitis is an Inflammatory Bowel Disease (IBD) that affects the large bowel (colon and rectum). It isn’t always as well-recognised as Crohn’s Disease or Ulcerative Colitis, other forms of IBD. It can take to time to get a diagnosis of Microscopic Colitis. And dealing with watery poo, tummy cramps, and rushing to the toilet can all be hard to manage. But you’re not alone; we’re here to support you. Many people respond well to treatment and often symptoms go away when you find what’s right for you. If you’ve just been diagnosed or want to know more about Microscopic Colitis, this information is for you. WHAT IS MICROSCOPIC COLITIS Microscopic Colitis affects the part of the gut known as the large bowel – the colon and rectum. The large bowel, or colon’s main job is to absorb water from your poo, so you can pass a solid but soft and well-formed poo. THE GUT 1 OESOPHAGUS 2 STOMACH 1 3 SMALL BOWEL 2 4 COLON LARGE BOWEL 5 RECTUM} 6 ANUS 2 4 3 5 6 1 Crohn’s & Colitis UK | www.crohnsandcolitis.org.uk MICROSCOPIC COLITIS The walls of your bowel have layers. In Microscopic Colitis the inner lining becomes inflamed. But this change can only be seen when a sample of tissue (biopsy) is taken from your colon and looked at under a microscope. BOWEL LAYERS INNER LINING MIDDLE LAYER OUTER LAYER There are two types of Microscopic Colitis: • Lymphocytic Colitis (LC) – where the inner lining has more white blood cells (lymphocytes) than usual. -

European Consensus on the Histopathology of Inflammatory Bowel Disease☆ F
Journal of Crohn's and Colitis (2013) 7, 827–851 Available online at www.sciencedirect.com ScienceDirect CONSENSUS/GUIDELINES European consensus on the histopathology of inflammatory bowel disease☆ F. Magro a,⁎,1, C. Langner b,1, A. Driessen c, A. Ensari d, K. Geboes e, G.J. Mantzaris f, V. Villanacci g, G. Becheanu h, P. Borralho Nunes i, G. Cathomas j, W. Fries k, A. Jouret-Mourin l, C. Mescoli m, G. de Petris n, C.A. Rubio o, N.A. Shepherd p, M. Vieth q, R. Eliakim r on behalf of the European Society of Pathology (ESP) and the European Crohn's and Colitis Organisation (ECCO)2 a Department of Pharmacology & Therapeutics, Institute for Molecular and Cell Biology, Faculty of Medicine University of Porto, Department of Gastroenterology, Hospital de Sao Joao, Porto, Portugal b Institute of Pathology, Medical University of Graz, Austria c Department of Pathology, University Hospital Antwerp, Belgium d Department of Pathology, Ankara University Medical School, Turkey e Department of Pathology, UZ Leuven, Belgium f 1st Department of Gastroenterology, Evangelismos Hospital, Athens, Greece g Pathology, Spedali Civili, Brescia, Italy h Carol Davila University of Medicine and Pharmacy, Department of Pathology, Bucharest, Romania i Instituto de Anatomia Patologica, Escola Superior de Tecnologia da Saúde de Lisboa & Faculdade de Medicina da Universidade de Lisboa, Portugal j Institute for Pathology, Kanonsspital Baselland, Liestal, Switzerland k Dept. of Clinical and Experimental Medicine, Clinical Unit for Chronic Bowel Disorders, University of -

Diverticular Disease-Related Colitis
Diverticular Disease-Related Colitis KEY FACTS Colon TERMINOLOGY ○ Abscess, fistula, perforation • Segmental colitis-associated diverticulosis (SCAD) ○ Exception is Crohn disease-like variant of SCAD that may show mural lymphoid aggregates ETIOLOGY/PATHOGENESIS MICROSCOPIC • Unknown, TNF-α may play role • Chronic colitis-like changes mimicking inflammatory bowel CLINICAL ISSUES disease • Presents with hematochezia, abdominal pain, diarrhea • Ulcerative colitis-like variant shows changes confined to • Median age: 64 years mucosa ○ Range: 40-86 years ○ Diverticulitis may or may not be present in these cases • Predominately involves descending and sigmoid colon (with • Crohn disease-like variant shows mural lymphoid rectal sparing) aggregates • Treatment directed toward diverticular disease suppresses • Changes in both variants confined to segment involved symptoms with diverticulosis coli MACROSCOPIC TOP DIFFERENTIAL DIAGNOSES • Mucosal changes are mild and nonspecific • Ulcerative colitis, Crohn disease, infectious colitis, diversion • Mural changes are related more to underlying diverticulosis colitis, NSAID-associated colitis coli rather than SCAD Diverticular Disease-Associated Colitis Diverticular Disease-Associated Colitis (Left) The mucosa surrounding the openings of diverticula ſt into the colonic lumen is erythematous and granular, consistent with diverticular disease-associated colitis (DDAC) . (Right) It is not uncommon to find some inflammation or erosions around the luminal opening of a colonic diverticulum ſt. To be diagnostic of DDAC, inflammation must involve the mucosa in the interdiverticular region . Chronic Active Colitis Basal Lymphoplasmacytosis (Left) A chronic colitis pattern of inflammatory infiltrate is seen in both the ulcerative colitis-like and Crohn disease- like variant of DDAC. The mucosal changes are indistinguishable from true inflammatory bowel disease (IBD). (Right) A band of lymphoplasmacytic infiltrate is present beneath the base of the crypts in the mucosa.