Kenneth Fine, M.D. Individuals – Can Become Autoimmune
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Microscopic Colitis: Collagenous Colitis and Lymphocytic Colitis
Microscopic Colitis: Collagenous Colitis and Lymphocytic Colitis National Digestive Diseases Information Clearinghouse What is microscopic colitis? The term bowel refers to any part of the small or large intestine. The large intes- Microscopic colitis is inflammation of the tine includes the colon and the rectum, and bowel that is only visible using a microscope. together they are about 5 feet long. The Microscopic colitis is a type of inflammatory U.S. Department small intestine can be 12 to 20 feet long. bowel disease (IBD), which refers to a group of Health and Colitis means inflammation of the colon. Human Services of conditions that causes inflammation in the Microscopic colitis is inflammation of the bowel due to an excessive build-up of white colon and rectum. NATIONAL blood cells in the bowel lining. Microscopic INSTITUTES OF HEALTH colitis is less severe than other types of IBD because it does not lead to cancer and rarely What are collagenous colitis requires surgery. However, microscopic and lymphocytic colitis? colitis can cause considerable pain and Microscopic colitis has two main forms: discomfort. collagenous colitis and lymphocytic colitis. The symptoms of and treatment for both are identical. Some scientists believe the two forms may be different presentations of the same disease. Slight differences in the way intestinal tissues appear when seen with a microscope set them apart. In both forms, an increase in white blood cells can be seen within the intestinal epithelium—the layer of Stomach Liver cells that lines the intestine. Increased white blood cells are a sign of inflammation. But with collagenous colitis, the layer of collagen Colon (shaded) beneath the epithelium appears thicker than normal. -

Microscopic Colitis
From Department of Medicine, Solna Karolinska Institutet, Stockholm, Sweden MICROSCOPIC COLITIS Marie-Rose Mellander Stockholm 2017 All previously published papers were reproduced with permission from the publisher. Published by Karolinska Institutet. Printed by Eprint AB 2017 © Marie-Rose Mellander, 2017 ISBN 978-91-7676-586-9 Microscopic colitis THESIS FOR DOCTORAL DEGREE (Ph.D.) By Marie-Rose Mellander Principal Supervisor: Opponent: Rolf Hultcrantz Marie Carlson Karolinska Institutet Uppsala University Department of Medicine, Huddinge Department of Medical Sciences; Gastroenterology/Hepatology Co-supervisors: Jan Björk Examination Board: Karolinska Institutet Catarina Almqvist Malmros Department of Medicine, Solna Karolinska Institutet Department of Medical Epidemiology and Robert Löfberg Biostatistics Karolinska Institutet Department of Medicine, Solna Kjell-Arne Ung University of Gothenburg Anders Ekbom Department of Medicine, Sahlgrenska Karolinska Institutet Department of Medicine, Solna Anders Höög Karolinska Institutet Department of Oncology-Pathology To my daughters Julia, Tilda and Sara ABSTRACT Microscopic colitis (MC) is an inflammatory bowel disease (IBD) and a common cause of chronic non-bloody diarrhoea, especially in elderly women. There are two main subtypes, lymphocytic colitis (LC) and collagenous colitis (CC) which are clinically indistinguishable and can be separated only by their characteristic histopathological features. The colonoscopy is usually macroscopically normal although subtle mucosal changes have been reported. The aetiology of MC is unknown and the genetic factors are poorly investigated. This thesis aims to describe MC in a large urban cohort and compare LC and CC regarding clinical and endoscopic features, both at diagnosis and at follow-up (F-U), and to observe the occurrence of coeliac disease, ulcerative colitis (UC) and Crohn’s disease (CD). -
Microscopic Colitis Collagenous and Lymphocytic Colitis Publisher
The informed patient Microscopic colitis Collagenous and lymphocytic colitis Publisher © 2020 Dr. Falk Pharma GmbH 6th updated and All rights reserved. revised edition 2020 The informed patient Microscopic colitis Collagenous and lymphocytic colitis Prof. Dr. Andreas Tromm, Hattingen (Germany) Microscopic colitis – Collagenous and lymphocytic colitis Address of the author Prof. Dr. Andreas Tromm Klinik für Innere Medizin Augusta-Kranken-Anstalt gGmbH Betriebsstelle EVK Hattingen Akademisches Lehrkrankenhaus der Universität Duisburg-Essen Bredenscheider Str. 54 45525 Hattingen Germany www.klinik-gastroenterologie.de Contents Introduction 4 Clinical presentation 6 Causes and development of microscopic colitis 9 Diagnosis 11 Treatment 14 Frequently asked questions about microscopic colitis 17 3 Microscopic colitis – Collagenous and lymphocytic colitis Introduction The term microscopic colitis encompasses two different disorders of the colon known as collagenous colitis and lymphocytic colitis. Both disorders are characterized by non-bloody watery diarrhea and are sometimes referred to as watery diar- rhea syndromes (Fig. 1). Microscopic colitis Collagenous colitis Lymphocytic colitis Watery diarrhea syndrome Fig. 1: Definition of microscopic colitis. 4 Introduction The term microscopic colitis describes a chronic in- flammatory disease of the colon (“colitis” comes from the Latin term “colon” and the ending “-itis”, which is used in medicine to refer to inflammation) that a doctor cannot identify by colonoscopy with the naked eye because the mucosa of the colon appears normal. In order for the disease to be diagnosed, the doctor must therefore remove a small tissue sample and examine it under a microscope. This is the only way to diagnose microscopic colitis. For collagenous colitis, a thickened collagen layer becomes visible when the tissue samples are stained using special methods, whereas lymphocytic colitis is detected as an increased number of a specific type of white blood cells called lymphocytes (see page 12). -

Microscopic Colitis What Is Microscopic Colitis?
Microscopic Colitis What is microscopic colitis? Microscopic colitis is an inflammation of the colon that a health care provider can see only with a microscope. Inflammation is the body’s normal response to injury, irritation, or infection of tissues. Microscopic colitis is a type of inflammatory bowel disease—the general name for diseases that cause irritation and inflammation in the intestines. The two types of microscopic colitis are collagenous colitis and lymphocytic colitis. Health care providers often use the term microscopic colitis to describe both types because their symptoms and treatments are the same. Some scientists believe that collagenous colitis and lymphocytic colitis may be different phases of the same condition rather than separate conditions. In both types of microscopic colitis, an increase in the number of lymphocytes, a type of white blood cell, can be seen in the epithelium—the layer of cells that lines the colon. An increase in the number of white blood cells is a sign of inflammation. The two types of colitis affect the colon tissue in slightly different ways: Lymphocytic colitis. The number of lymphocytes is higher, and the tissues and lining of the colon are of normal thickness. Collagenous colitis. The layer of collagen, a threadlike protein, underneath the epithelium builds up and becomes thicker than normal. When looking through a microscope, the health care provider may find variations in lymphocyte numbers and collagen thickness in different parts of the colon. These variations may indicate an overlap of the two types of microscopic colitis. What causes microscopic colitis? The exact cause of microscopic colitis is unknown. -

Lymphocytic Colitis: a Retrospective Clinical Study of 199 Gut: First Published As 10.1136/Gut.2003.023440 on 11 March 2004
536 INFLAMMATION Lymphocytic colitis: a retrospective clinical study of 199 Gut: first published as 10.1136/gut.2003.023440 on 11 March 2004. Downloaded from Swedish patients M Olesen, S Eriksson, J Bohr, G Ja¨rnerot, C Tysk ............................................................................................................................... Gut 2004;53:536–541. doi: 10.1136/gut.2003.023440 Background: Lymphocytic colitis is characterised by chronic diarrhoea and specific microscopic changes in a macroscopically normal colonic mucosa. We report clinical features and treatment outcome in a large patient cohort. Methods: Patients were searched for in 24 Swedish gastroenterology clinics. The biopsy material was reassessed using strict histopathological criteria. Clinical data were obtained from medical notes. See end of article for Results: Lymphocytic colitis was diagnosed in 199 cases. The female:male ratio was 2.4:1. Median age at authors’ affiliations diagnosis was 59 (48–70) years. The most frequent symptoms were diarrhoea (96%), abdominal pain ....................... (47%), and weight loss (41%). The course was chronic intermittent in 30% of patients, chronic continuous in Correspondence to: 7%, and a single attack in 63%, and in these cases the disease duration was 6 (4–11) months. Seventy Associate Professor nine (40%) patients reported associated diseases, of which thyroid disorders, coeliac disease, and C Tysk, Department of diabetes mellitus were the most common. In 34 first or second degree relatives of 24 (12%) patients, a Medicine, Division of family history of ulcerative colitis, Crohn’s disease, collagenous colitis, or coeliac disease was reported. Gastroenterology, O¨ rebro University Hospital, Drug induced disease was suspected in 19 (10%) patients. A non-significant peak of disease onset was 701 85 O¨ rebro, Sweden; seen in December-January. -

What Is Colitis? Pitfalls in the Microscopic Diagnosis
What is colitis? Pitfalls in the microscopic diagnosis K. Geboes, KULeuven, Bucarest 2011 What is colitis? Statistical approach (morphometry)? • Chronic inflammatory infiltration total cellularity increase • Surface epithelial height to crypt epithelial height. In normal mucosa the surface epithelial cell height exceeds the height of crypt epithelium • Redistribution of infiltrating cells so that there is a similar density in the basal third to that of the superficial third > IBD Jenkins e.a. J Clin Pathol 1988; 41; 72-79 1 Normal mucosa vs Colitis – Lamina propria cellular infiltrate : increase in intensity; composition & distribution – Organized lymphoid tissue : increase, stimulation – Epithelial cells : • surface epithelium – terminally differentiated cells DAMAGE & REPAIR (restitution) • crypts – differentiating cells, proliferative compartment INCREASED PROLIFERATION (mitotic activity) • normal turnover : increased turnover Basic lesions : Inflammation • Inflammation pattern I – Patchy, focal – Diffuse 2 Basic lesions : Inflammation • Inflammation pattern II – Diffuse upper third (Infections such as Shigella colitis) – Diffuse transmucosal (IBD) Basic lesions : Architecture • Surface – Flat or irregular 3 Basic lesions : Architecture • Crypt architecture – Crypt density • 7/8 crypts per 1 mm mucosal length (IBD 4 to 5) • Closely packed – Variable or constant intercryptal distance Basic lesions : Architecture • Crypt architecture – Straight or branching tubes (infrequent branching < 10% may be normal) – Base reaching muscularis mucosae -

Advances in Digestive Diseases 2019: Highlights from DDW 6/1/19 Richard Kalman, MD Director Liver Tumor Program [email protected] Outline
Recent Advances in Autoimmune Gastrointestinal Disorders Advances in Digestive Diseases 2019: Highlights from DDW 6/1/19 Richard Kalman, MD Director Liver Tumor Program [email protected] Outline • Celiac Disease • Microscopic Colitis • Autoimmune Hepatitis • IBD • EoE • Autoimmune gastritis • Autoimmune enteropathy • PBC • PSC Richard Kalman [email protected] Outline • Celiac Disease • Microscopic Colitis • Autoimmune Hepatitis • IBD • EoE • Autoimmune gastritis • Autoimmune enteropathy • PBC • PSC Richard Kalman [email protected] Celiac Disease Richard Kalman [email protected] Celiac Disease • Immune mediated small intestine enteropathy • Partial gluten digestion • Bind HLA DQ on APCs – Activate CD4+ T cells • Proinflammatory cytokine • Stimulates B cell response – Promote IEL activation • Transform to NK like cells • Destroy enterocytes Tye-Din JA et al. Front Pediat. 2018 Celiac Disease • Injury → less absorptive area → reduction in digestive enzymes → micronutrient deficiencies – fat soluble vitamins, iron, B12, Folic acid • Inflammation exacerbates malabsorption by causing secretion of fluid that increases diarrhea • 100% of patients possess specific variants of HLA class II genes - HLA-DQ2 and DQ8 – 90% DQ2 – 20-30% of US population carry these genes– only 2- 3% of these people will get CD Lebwohol B et al. Lancet. 2018 Celiac Disease • Presentation – Asymptomatic to severe malnutrition – GI – pain, bloating, diarrhea, dyspepsia, weight loss, steatorrhea – Extra-intestinal – fatigue, joint pain, cognitive -
Microscopic Colitis (PDF)
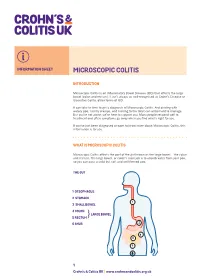
INFORMATION SHEET MICROSCOPIC COLITIS INTRODUCTION Microscopic Colitis is an Inflammatory Bowel Disease (IBD) that affects the large bowel (colon and rectum). It isn’t always as well-recognised as Crohn’s Disease or Ulcerative Colitis, other forms of IBD. It can take to time to get a diagnosis of Microscopic Colitis. And dealing with watery poo, tummy cramps, and rushing to the toilet can all be hard to manage. But you’re not alone; we’re here to support you. Many people respond well to treatment and often symptoms go away when you find what’s right for you. If you’ve just been diagnosed or want to know more about Microscopic Colitis, this information is for you. WHAT IS MICROSCOPIC COLITIS Microscopic Colitis affects the part of the gut known as the large bowel – the colon and rectum. The large bowel, or colon’s main job is to absorb water from your poo, so you can pass a solid but soft and well-formed poo. THE GUT 1 OESOPHAGUS 2 STOMACH 1 3 SMALL BOWEL 2 4 COLON LARGE BOWEL 5 RECTUM} 6 ANUS 2 4 3 5 6 1 Crohn’s & Colitis UK | www.crohnsandcolitis.org.uk MICROSCOPIC COLITIS The walls of your bowel have layers. In Microscopic Colitis the inner lining becomes inflamed. But this change can only be seen when a sample of tissue (biopsy) is taken from your colon and looked at under a microscope. BOWEL LAYERS INNER LINING MIDDLE LAYER OUTER LAYER There are two types of Microscopic Colitis: • Lymphocytic Colitis (LC) – where the inner lining has more white blood cells (lymphocytes) than usual. -

Diverticular Disease-Related Colitis
Diverticular Disease-Related Colitis KEY FACTS Colon TERMINOLOGY ○ Abscess, fistula, perforation • Segmental colitis-associated diverticulosis (SCAD) ○ Exception is Crohn disease-like variant of SCAD that may show mural lymphoid aggregates ETIOLOGY/PATHOGENESIS MICROSCOPIC • Unknown, TNF-α may play role • Chronic colitis-like changes mimicking inflammatory bowel CLINICAL ISSUES disease • Presents with hematochezia, abdominal pain, diarrhea • Ulcerative colitis-like variant shows changes confined to • Median age: 64 years mucosa ○ Range: 40-86 years ○ Diverticulitis may or may not be present in these cases • Predominately involves descending and sigmoid colon (with • Crohn disease-like variant shows mural lymphoid rectal sparing) aggregates • Treatment directed toward diverticular disease suppresses • Changes in both variants confined to segment involved symptoms with diverticulosis coli MACROSCOPIC TOP DIFFERENTIAL DIAGNOSES • Mucosal changes are mild and nonspecific • Ulcerative colitis, Crohn disease, infectious colitis, diversion • Mural changes are related more to underlying diverticulosis colitis, NSAID-associated colitis coli rather than SCAD Diverticular Disease-Associated Colitis Diverticular Disease-Associated Colitis (Left) The mucosa surrounding the openings of diverticula ſt into the colonic lumen is erythematous and granular, consistent with diverticular disease-associated colitis (DDAC) . (Right) It is not uncommon to find some inflammation or erosions around the luminal opening of a colonic diverticulum ſt. To be diagnostic of DDAC, inflammation must involve the mucosa in the interdiverticular region . Chronic Active Colitis Basal Lymphoplasmacytosis (Left) A chronic colitis pattern of inflammatory infiltrate is seen in both the ulcerative colitis-like and Crohn disease- like variant of DDAC. The mucosal changes are indistinguishable from true inflammatory bowel disease (IBD). (Right) A band of lymphoplasmacytic infiltrate is present beneath the base of the crypts in the mucosa. -
Microscopic Colitis Collagenous and Lymphocytic Colitis
The informed patient Microscopic colitis Collagenous and lymphocytic colitis A. Tromm, Evangelisches Krankenhaus Hattingen (Germany) 2-11/2010/3.000 Burger Bu82e Publisher © 2017 Falk Foundation e.V. All rights reserved. 4th revised edition 2017 The informed patient Microscopic colitis Collagenous and lymphocytic colitis A. Tromm, Evangelisches Krankenhaus Hattingen (Germany) Author: Prof. Dr. A. Tromm Klinik für Innere Medizin Evangelisches Krankenhaus Hattingen gGmbH Akademisches Lehrkrankenhaus der Universität Duisburg-Essen Bredenscheider Str. 54 45525 Hattingen Germany www.klinik-gastroenterologie.de The informed patient Contents Page Introduction 5 Clinical manifestations 7 Causes and pathogenesis of microscopic colitis 10 Diagnostics 12 Treatment 15 Collagenous colitis 15 Lymphocytic colitis 18 Frequently asked questions about microscopic colitis 19 3 The informed patient Introduction The term microscopic colitis encompasses two different disorders of the colon known as colla- genous colitis and lymphocytic colitis. Both disorders are characterized by watery diar- rhea and are therefore also known as watery diar- rhea syndrome (Figure 1). microscopic colitis collagenous lymphocytic colitis colitis watery diarrhea syndrome Figure 1: Definition of microscopic colitis 5 The term microscopic colitis refers to an inflam- matory disorder of the colon (colitis) which can- not be recognized with the naked eye during an endoscopy because the bowel mucosa appears normal. Small tissue samples are taken and ex- amined under the microscope. Microscopic colitis can only be diagnosed microscopically. In collagenous colitis, special staining of the tis- sue samples reveals a thickened collagen band, whereas lymphocytic colitis is typified by an in- creased number of lymphocytes, a type of white blood cell. Since the 1980s, when microscopic colitis was first described, knowledge about this disorder has increased greatly. -

Microscopic Colitis—Microbiome, Barrier Function and Associated Diseases
Review Article Page 1 of 10 Microscopic colitis—microbiome, barrier function and associated diseases Saskia van Hemert1, Karolina Skonieczna-Żydecka2, Igor Loniewski2,3, Piotr Szredzki4, Wojciech Marlicz5 1Winclove Probiotics, Amsterdam, the Netherlands; 2Department of Biochemistry and Human Nutrition, Pomeranian Medical University, Szczecin, Poland; 3Sanprobi Sp. z o.o. Sp. K., Szczecin, Poland; 4Endoscopy Unit, Department of Surgery, Hospital Sędziszów Małopolski, Sędziszów Małopolski, Poland; 5Department of Gastroenterology, Pomeranian Medical University, Szczecin, Poland Contributions: (I) Conception and design: S van Hemert, W Marlicz; (II) Administrative support: None; (III) Provision of study materials or patients: K Żydecka-Skonieczna, I Loniewski; (IV) Collection and assembly of data: S van Hemert, K Żydecka-Skonieczna, W Marlicz; (V) Data analysis and interpretation: All authors; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Wojciech Marlicz, MD, PhD, FACG. Department of Gastroenterology, Pomeranian Medical University, Unii Lubelskiej 1, 71-252 Szczecin, Poland. Email: [email protected]. Abstract: Microscopic colitis (MC) is a chronic inflammatory bowel disease (IBD) with little in terms of endoscopic abnormalities and is frequently associated with other autoimmune diseases. The peak incidence of the disease is in middle aged or older populations, mostly females. The pathogenesis of MC is complex, multifactorial and poorly understood. Current concepts revolve around innate immunity or microbiome alterations as well as gut barrier dysfunction, all of which lead to the development of subtle inflammatory lesions in gut mucosa. The results of numerous basic and clinical studies involving molecular techniques as well as advanced endoscopic imaging revealed the important role of both intrinsic (e.g., hormonal) as well as extrinsic (e.g., NSAIDs and PPIs) factors in the modulation of gastrointestinal microbiome and MC pathogenesis. -

Undiagnosed Microscopic Colitis: a Hidden Cause of Chronic Diarrhoea and a Frequently Missed Treatment Opportunity
EDUCATION Frontline Gastroenterol: first published as 10.1136/flgastro-2019-101227 on 5 July 2019. Downloaded from REVIEW Undiagnosed microscopic colitis: a hidden cause of chronic diarrhoea and a frequently missed treatment opportunity Andreas Münch ,1 David S Sanders,2 Michael Molloy- Bland,3 A Pali S Hungin4 1Division of Gastroenterology ABSTRACT patients and clinicians. In many national and Hepatology, Department Microscopic colitis (MC) is a treatable cause of Clinical and Experimental settings, because of downward pressure Medicine, Faculty of Health of chronic, non-bloody , watery diarrhoea, but to minimise specialist referrals and colo- Science, Linköping University, physicians (particularly in primary care) are less noscopies with histology in patients with Linköping, Sweden familiar with MC than with other causes of 2Department of seemingly normal endoscopic appear- Gastroenterology, Royal chronic diarrhoea. The colon in patients with ances, the systems for diagnosing and Hallamshire Hospital, Sheffield, MC is usually macroscopically normal. MC can managing patients with gastrointestinal UK only be diagnosed by histological examination 3Oxford PharmaGenesis, problems work against a diagnosis of MC Melbourne, Victoria, Australia of colonic biopsies (subepithelial collagen band and its effective management. 4 The Institute of Health and >10 µm (collagenous colitis) or >20 intraepithelial When first described in 1980, MC was Society, Faculty of Medical lymphocytes per 100 epithelial cells (lymphocytic 1 Sciences, Newcastle University, thought to be a rare condition. We now Newcastle upon Tyne, UK colitis), both with lamina propria inflammation). know that MC is a common form of inflam- The UK National Health Service exerts downward matory bowel disease (IBD), with one Correspondence to pressure to minimise colonoscopy referrals.