Reactions of Alcohols - Part 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SAFETY DATA SHEET Isopropyl Alcohol
SAFETY DATA SHEET Isopropyl Alcohol Section 1. Identification GHS product identifier : Isopropyl Alcohol Chemical name : Isopropyl alcohol Other means of : isopropanol; 2-Propanol identification Product type : Liquid. Product use : Synthetic/Analytical chemistry. Synonym : isopropanol; 2-Propanol SDS # : 001105 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : FLAMMABLE LIQUIDS - Category 2 substance or mixture EYE IRRITATION - Category 2A SPECIFIC TARGET ORGAN TOXICITY (SINGLE EXPOSURE) (Narcotic effects) - Category 3 GHS label elements Hazard pictograms : Signal word : Danger Hazard statements : May form explosive mixtures with air. Highly flammable liquid and vapor. Causes serious eye irritation. May cause drowsiness or dizziness. Precautionary statements General : Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Prevention : Wear protective gloves. Wear eye or face protection. Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. Use explosion- proof electrical, ventilating, lighting and all material-handling equipment. Use only non- sparking tools. Take precautionary measures against static discharge. Keep container tightly closed. Use only outdoors or in a well-ventilated area. Avoid breathing vapor. Wash hands thoroughly after handling. Response : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Call a POISON CENTER or physician if you feel unwell. -

N-BUTYL ALCOHOL
Right to Know Hazardous Substance Fact Sheet Common Name: n-BUTYL ALCOHOL Synonyms: Propyl Carbinol; n-Butanol CAS Number: 71-36-3 Chemical Name: 1-Butanol RTK Substance Number: 1330 Date: November 1998 Revision: January 2008 DOT Number: UN 1120 Description and Use EMERGENCY RESPONDERS >>>> SEE BACK PAGE n-Butyl Alcohol is a colorless liquid with a strong, sweet Hazard Summary alcohol odor. It is used as a solvent for fats, waxes, shellacs, Hazard Rating NJDOH NFPA resins, gums, and varnish, in making hydraulic fluids, and in HEALTH - 2 medications for animals. FLAMMABILITY - 3 REACTIVITY - 0 f ODOR THRESHOLD = 1 to 15 ppm FLAMMABLE f Odor thresholds vary greatly. Do not rely on odor alone to POISONOUS GASES ARE PRODUCED IN FIRE determine potentially hazardous exposures. CONTAINERS MAY EXPLODE IN FIRE Hazard Rating Key: 0=minimal; 1=slight; 2=moderate; 3=serious; Reasons for Citation 4=severe f n-Butyl Alcohol is on the Right to Know Hazardous f n-Butyl Alcohol can affect you when inhaled and by Substance List because it is cited by OSHA, ACGIH, DOT, passing through the skin. NIOSH, DEP, IRIS, NFPA and EPA. f Contact can irritate and burn the skin. f This chemical is on the Special Health Hazard Substance f n-Butyl Alcohol can irritate and burn the eyes with possible List. eye damage. f Inhaling n-Butyl Alcohol can irritate the nose, throat and lungs. f Exposure to n-Butyl Alcohol can cause headache, dizziness, nausea and vomiting. SEE GLOSSARY ON PAGE 5. f n-Butyl Alcohol can damage the liver, kidneys, hearing, and sense of balance. -
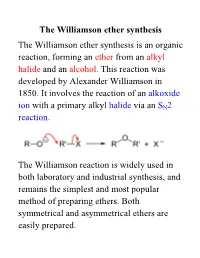
Williamson Ether Synthesis the Williamson Ether Synthesis Is an Organic Reaction, Forming an Ether from an Alkyl Halide and an Alcohol
The Williamson ether synthesis The Williamson ether synthesis is an organic reaction, forming an ether from an alkyl halide and an alcohol. This reaction was developed by Alexander Williamson in 1850. It involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction. The Williamson reaction is widely used in both laboratory and industrial synthesis, and remains the simplest and most popular method of preparing ethers. Both symmetrical and asymmetrical ethers are easily prepared. The reaction for this week: an example of a Williamson ether synthesis acetaminophen ethyl iodide phenacetin starting material reagent product Phenacetin may be synthesized as an example of the Williamson ether synthesis The first synthesis of phenacetin was reported in 1878 by Harmon Morse. Procedure 1. Weigh an Extra-Strength Tylenol tablet. Pulverize the tablet with mortar and pestle. Weigh out 0.22 g and place it in a dry 15-ml round-bottom flask along with 0.28 g of finely pulverized K2CO3 (mortar and pestle) and 3.0 mL of butanone. Carefully add 0.28 mL of ethyl iodide with a syringe. 2. Add a stir bar; attach a microscale water-cooled condenser to the flask. Heat the mixture under reflux directly on a hot plate at medium setting for 1 hour. In the meantime, obtain the IR of acetaminophen. 3. Turn off the heat. Allow the mixture to cool down. Add 4 mL of water to the flask and transfer its contents to a 16 x 125 mm test tube with a screw cap. Rinse round-bottom flask 4 times with 1 mL of tert-butyl methyl ether (BME) and add the rinsings to the test tube. -

How Is Alcohol Metabolized by the Body?
Overview: How Is Alcohol Metabolized by the Body? Samir Zakhari, Ph.D. Alcohol is eliminated from the body by various metabolic mechanisms. The primary enzymes involved are aldehyde dehydrogenase (ALDH), alcohol dehydrogenase (ADH), cytochrome P450 (CYP2E1), and catalase. Variations in the genes for these enzymes have been found to influence alcohol consumption, alcohol-related tissue damage, and alcohol dependence. The consequences of alcohol metabolism include oxygen deficits (i.e., hypoxia) in the liver; interaction between alcohol metabolism byproducts and other cell components, resulting in the formation of harmful compounds (i.e., adducts); formation of highly reactive oxygen-containing molecules (i.e., reactive oxygen species [ROS]) that can damage other cell components; changes in the ratio of NADH to NAD+ (i.e., the cell’s redox state); tissue damage; fetal damage; impairment of other metabolic processes; cancer; and medication interactions. Several issues related to alcohol metabolism require further research. KEY WORDS: Ethanol-to acetaldehyde metabolism; alcohol dehydrogenase (ADH); aldehyde dehydrogenase (ALDH); acetaldehyde; acetate; cytochrome P450 2E1 (CYP2E1); catalase; reactive oxygen species (ROS); blood alcohol concentration (BAC); liver; stomach; brain; fetal alcohol effects; genetics and heredity; ethnic group; hypoxia The alcohol elimination rate varies state of liver cells. Chronic alcohol con- he effects of alcohol (i.e., ethanol) widely (i.e., three-fold) among individ- sumption and alcohol metabolism are on various tissues depend on its uals and is influenced by factors such as strongly linked to several pathological concentration in the blood T chronic alcohol consumption, diet, age, consequences and tissue damage. (blood alcohol concentration [BAC]) smoking, and time of day (Bennion and Understanding the balance of alcohol’s over time. -

Phosphine-Catalyzed Additions of Nucleophiles and Electrophiles to Α
PHOSPHINE-CATALYZED ADDITIONS OF NUCLEOPHILES AND ELECTROPHILES TO α,β–UNSATURATED CARBONYL COMPOUNDS Reported by Michael Scott Bultman November 4, 2004 INTRODUCTION Organophosphorous compounds are becoming increasingly important in organic synthesis. Phosphines serve as precursors to phosphonium ylides in the Wittig reaction,1 and as nucleophilic triggers in the Mitsunobu2 and Staudinger3 reactions. In these processes, the phosphine is stoichiometrically consumed and converted into a phosphine oxide. Phosphines are also commonly used as ligands for transition metal-catalyzed reactions, to modulate reactivity and stereocontrol.4 On the other hand, the use of phosphines as nucleophilic catalysts for organic reactions has only gained attention in the last ten years. First reported by Rauhut and Currier in 1963,5 phosphine catalysis has since been reinvestigated after the phosphine ligands in some transition-metal-catalyzed reactions were found to be better catalysts than the metal/phosphine complexes alone!6 Phosphines are well suited for catalyzing the addition of both nucleophiles and electrophiles to electron deficient alkenes, alkynes, and allenes. Activation of these α,β-unsaturated carbonyl systems with the phosphine enables the formation of new bonds at the α-, β-, and γ-positions. This report will highlight these different modes of addition to α,β-unsaturated carbonyl systems under phosphine catalysis that allow for the formation of a wide array of products from a single class of substrates. GENERAL REACTIVITY OF PHOSPHINES Key characteristics required for successful nucleophilic catalysis lie in the balance of leaving group ability, nucleophilicity, and ease of ylid formation. Increasing leaving group ability can often be + correlated with decreasing basicity. -

Sugar Alcohols—Their Role in the Modern World of Sweeteners: a Review
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Springer - Publisher Connector Eur Food Res Technol (2015) 241:1–14 DOI 10.1007/s00217-015-2437-7 REVIEW PAPER Sugar alcohols—their role in the modern world of sweeteners: a review Małgorzata Grembecka Received: 31 August 2014 / Revised: 16 December 2014 / Accepted: 13 February 2015 / Published online: 28 February 2015 © The Author(s) 2015. This article is published with open access at Springerlink.com Abstract Epidemic obesity and diabetes encouraged the observed. It was mainly due to the developments in bio- changes in population lifestyle and consumers’ food prod- logical studies, the change of a population lifestyle and the ucts awareness. Food industry has responded people’s increase in the consumer awareness concerning food prod- demand by producing a number of energy-reduced prod- ucts. The health quality of food depends mainly on nutri- ucts with sugar alcohols as sweeteners. These compounds ents, but also on foreign substances such as food additives. are usually produced by a catalytic hydrogenation of carbo- The presence of foreign substances in the food can be justi- hydrates, but they can be also found in nature in fruits, veg- fied, allowed or tolerated only when they are harmless to etables or mushrooms as well as in human organism. Due our health. Epidemic obesity and diabetes encouraged the to their properties, sugar alcohols are widely used in food, growth of the artificial sweetener industry. There are more beverage, confectionery and pharmaceutical industries and more people who are trying to lose weight or keeping throughout the world. -

Core Alcohol and Drug Survey
Form 194 Core Alcohol and Drug Survey For additional use: Long Form A 0 1 2 3 4 5 6 7 8 9 B 0 1 2 3 4 5 6 7 8 9 FIPSE Core Analysis Grantee Group Core Institute C 0 1 2 3 4 5 6 7 8 9 Student Health Programs 0 1 2 3 4 5 6 7 8 9 Southern Illinois University D Please use a number 2 Pencil. Carbondale, IL 62901 E 0 1 2 3 4 5 6 7 8 9 1. Classification: 2. Age: 3. Ethnic origin: 4. Marital status: Freshman . American Indian/ Single . Sophomore . Alaskan Native . Married . Junior . 0 0 Hispanic . Separated . Senior. 1 1 Asian/Pacific Islander . Divorced. Grad/professional . 2 2 White (non-Hispanic) . Widowed . Not seeking a 3 3 Black (non-Hispanic) . degree . 4 4 Other . 7. Are you working? Other . 5 5 Ye s, full-time . 6 6 6. Is your current residence Ye s, part-time . 5. Gender: 7 7 as a student: No . Male . 8 8 On-campus . Female . 9 9 Off-campus . 8. Living arrangements: A. Where: (mark best answer) 9. Approximate cumulative grade point average: (choose one) House/apartment/etc. Residence hall . A+AA- B+BB- C+CC- D+DD-F Approved housing. Fraternity or sorority . 10. Some students have indicated that alcohol or drug use at parties they attend in and Other . around campus reduces their enjoyment, often leads to negative situations, and therefore, they would rather not have alcohol and drugs available and used. Other B. With whom: students have indicated that alcohol and drug use at parties increases their (mark all that apply) enjoyment, often leads to positive situations, and therefore, they would rather have With roommate(s). -

Sugar Alcohols: a Review
International Journal of PharmTech Research CODEN (USA): IJPRIF, ISSN: 0974-4304, ISSN(Online): 2455-9563 Vol.9, No.7, pp 407-413, 2016 Sugar alcohols: A review Ramezan Ali Mahian1, Vahid Hakimzadeh2* 1,2Department of Food Science and Technology, Quchan Branch, Islamic Azad University, Quchan, Iran Abstract : Sugar alcohols are extensively used as sweetening agents. They sometimes possess advantages over the parent sugars in sweetness, caloric reduction and non-cariogenicity. The physical status of carbohydrates in food and confectionery affects both the properties of the product during production and the quality of the final product. Sugar alcohols, such as xylitol, mannitol, sorbitol, and erythritol are emerging food ingredients that provide similar or better sweetness/sensory properties of sucrose, but are less calorigenic. Also, sugar alcohols can be converted into commodity chemicals through chemical catalysis. Biotechnological production offers the safe and sustainable supply of sugar alcohols from renewable biomass. These compounds are usually produced by a catalytic hydrogenation of carbohydrates, but they can be also found in nature in fruits, vegetables or mushrooms as well as in human organism. Due to their properties, sugar alcohols are widely used in food, beverage, confectionery and pharmaceutical industries throughout the world. They have found use as bulk sweeteners that promote dental health and exert prebiotic effect. They are added to foods as alternative sweeteners what might be helpful in the control of calories intake. Consumption of low-calorie foods by the worldwide population has dramatically increased, as well as health concerns associated with the consequent high intake of sweeteners. Key words: Sugar alcohols, low-calorie, Sugar-free products, sweetener. -

Gut Microbiota Prevents Sugar Alcohol-Induced Diarrhea
nutrients Article Gut Microbiota Prevents Sugar Alcohol-Induced Diarrhea Kouya Hattori 1,2 , Masahiro Akiyama 1, Natsumi Seki 1,2, Kyosuke Yakabe 1,2, Koji Hase 2 and Yun-Gi Kim 1,* 1 Research Center for Drug Discovery, Faculty of Pharmacy and Graduate School of Pharmaceutical Sciences, Keio University, Tokyo 105-8512, Japan; [email protected] (K.H.); [email protected] (M.A.); [email protected] (N.S.); [email protected] (K.Y.) 2 Department of Biochemistry, Faculty of Pharmacy and Graduate School of Pharmaceutical Sciences, Keio University, Tokyo 105-8512, Japan; [email protected] * Correspondence: [email protected] Abstract: While poorly-absorbed sugar alcohols such as sorbitol are widely used as sweeteners, they may induce diarrhea in some individuals. However, the factors which determine an individual’s susceptibility to sugar alcohol-induced diarrhea remain unknown. Here, we show that specific gut bacteria are involved in the suppression of sorbitol-induced diarrhea. Based on 16S rDNA analysis, the abundance of Enterobacteriaceae bacteria increased in response to sorbitol consumption. We found that Escherichia coli of the family Enterobacteriaceae degraded sorbitol and suppressed sorbitol-induced diarrhea. Finally, we showed that the metabolism of sorbitol by the E. coli sugar phosphotransferase system helped suppress sorbitol-induced diarrhea. Therefore, gut microbiota prevented sugar alcohol-induced diarrhea by degrading sorbitol in the gut. The identification of the gut bacteria which respond to and degrade sugar alcohols in the intestine has implications for microbiome science, processed food science, and public health. Keywords: gut microbiota; sugar alcohol; diarrhea Citation: Hattori, K.; Akiyama, M.; Seki, N.; Yakabe, K.; Hase, K.; Kim, Y.-G. -

Sugar Alcohols Fact Sheet
International Food Information Council Sugar Alcohols Fact Sheet September 2004 BACKGROUND Sugar alcohols or polyols, as they are also called, have a long history of use in a wide variety of foods. Recent technical advances have added to the range of sugar alcohols available for food use and expanded the applications of these sugar replacers in diet and health-oriented foods. They have been found useful in sugar-free and reduced-sugar products, in foods intended for individuals with diabetes, and most recently in new products developed for carbohydrate controlled eating plans. Sugar alcohols are neither sugars nor alcohols. They are carbohydrates with a chemical structure that partially resembles sugar and partially resembles alcohol, but they don’t contain ethanol as alcoholic beverages do. They are incompletely absorbed and metabolized by the body, and consequently contribute fewer calories. The polyols commonly used include sorbitol, mannitol, xylitol, maltitol, maltitol syrup, lactitol, erythritol, isomalt and hydrogenated starch hydrolysates. Their calorie content ranges from 1.5 to 3 calories per gram compared to 4 calories per gram for sucrose or other sugars. Most are approximately half as sweet as sucrose; maltitol and xylitol are about as sweet as sucrose. Sugar alcohols occur naturally in a wide variety of fruits and vegetables, but are commercially produced from other carbohydrates such as sucrose, glucose, and starch. Along with adding a sweet taste, polyols perform a variety of functions such as adding bulk and texture, providing a cooling effect or taste, inhibiting the browning that occurs during heating and retaining moisture in foods. While polyols do not actually prevent browning, they do not cause browning either. -

Alcohol Fact Sheet
Alcohol Alcohol is a commonly used drug. There are two different types of alcohol. Ethyl alcohol (or ethanol) is made when vegetables, fruits and grains break down and go through a chemical process called fermentation. This type of alcohol is found in alcoholic drinks such as beer, wine, hard liquor or spirits. Methyl alcohol (or methanol) is found in household and industrial products like antifreeze and paint removers. This type of alcohol is poisonous to drink. Most people that drink alcohol do so in moderation. 5% alcohol per volume to 40% alcohol per volume) Alcohol is sometimes consumed with meals, used and are served in different sizes of containers (from in social gatherings and as part of celebrations. 1.5 oz to 12 oz), but they are all equal in terms of However, drinking too much alcohol or drinking alcohol content. Knowing this definition can help alcohol too often may affect the quality of your life. you make informed decisions about the amount of alcohol you consume. What is a standard drink? Short-Term Effects A standard drink can help you monitor and track how much alcohol you are drinking or planning on Alcohol slows your brain activity and impairs the drinking. way you think, speak and move. When you drink Each of the drinks pictured is considered a standard too much alcohol, you may make poor decisions drink. They have different strengths of alcohol (from and do things that you would not normally do. You may have slurred speech, blurred vision and trouble Standard Drink Each of theseStandard drinks has the same Drink amount of alcohol. -

And Isopropyl Alcohol for Methanol, Including During the Public Health Emergency (COVID-19)
Contains Nonbinding Recommendations Policy for Testing of Alcohol (Ethanol) and Isopropyl Alcohol for Methanol, Including During the Public Health Emergency (COVID-19) Guidance for Industry January 2021 U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) Center for Veterinary Medicine (CVM) Current Good Manufacturing Practice (CGMP) Contains Nonbinding Recommendations Preface Public Comment This guidance is being issued to address the Coronavirus Disease 2019 (COVID-19) public health emergency. This guidance is being implemented without prior public comment because the Food and Drug Administration (FDA or the Agency) has determined that prior public participation for this guidance is not feasible or appropriate (see section 701(h)(1)(C) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) and 21 CFR 10.115(g)(2)). This guidance document is being implemented immediately, but it remains subject to comment in accordance with the Agency’s good guidance practices. Comments may be submitted at any time for Agency consideration. Submit written comments to the Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852. Submit electronic comments to https://www.regulations.gov. All comments should be identified with the docket number FDA-2020-D-2016 and complete title of the guidance in the request. Additional Copies Additional copies are available from the FDA webpage