Catalytic Deoxydehydration of Glycerol to Allyl Alcohol – a Promising Pathway to a High-Versatile Platform Molecule
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alcohols Combined 1405
ALCOHOLS COMBINED 1405 Formulas: Table 1 MW: Table 1 CAS: Table 2 RTECS: Table 2 METHOD: 1405, Issue 1 EVALUATION: PARTIAL Issue 1: 15 March 2003 OSHA : Table 2 PROPERTIES: Table 1 NIOSH: Table 2 ACGIH: Table 2 COMPOUNDS: (1) n-butyl alcohol (4) n-propyl alcohol (7) cyclohexanol (2) sec-butyl alcohol (5) allyl alcohol (8) isoamyl alcohol (3) isobutyl alcohol (6) diacetone alcohol (9) methyl isobutyl carbinol SYNONYMS: See Table 3. SAMPLING MEASUREMENT SAMPLER: SOLID SORBENT TUBE TECHNIQUE: GAS CHROMATOGRAPHY, FID (Coconut shell charcoal, 100 mg/50 mg) ANALYTE: Compounds above FLOW RATE: 0.01 to 0.2 L/min DESORPTION: 1 mL 5% 2-propanol in CS2 Compounds: (1-3 ) (4-9) VOL-MIN: 2 L 1 L INJECTION -MAX: 10 L 10 L VOLUME: 1 µL SHIPMENT: Routine TEMPERATURE -INJECTION: 220 °C SAMPLE -DETECTOR: 250 - 300 °C STABILITY: See Evaluation of Method. -COLUMN: 35 °C (7 minutes), to 60 °C at 5 °C/minute, hold 5 minutes, up to BLANKS: 2 to 10 field blanks per set 120 °C at 10 °C /minute, hold 3 minutes. CARRIER GAS: He, 4 mL/min ACCURACY COLUMN: Capillary, fused silica, 30 m x 0.32-mm RANGE STUDIED: Not studied [1, 2]. ID; 0.5 µm film polyethylene glycol, DB- wax or equivalent BIAS: Not determined CALIBRATION: Solutions of analyte in eluent (internal OVERALL standard optional) PRECISION (Ö ): Not determined rT RANGE: See EVALUATION OF METHOD. ACCURACY: Not determined ESTIMATED LOD: 1 µg each analyte per sample PRECISION: See EVALUATION OF METHOD. APPLICABILITY: This method may be used to determine two or more of the specified analytes simultaneously. -
Chapter 13.Pptx
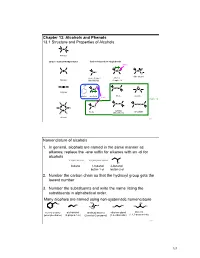
Chapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols C C Alkanes Carbon - Carbon Multiple Bonds Carbon-heteroatom single bonds basic O C C C N C N C X O nitro alkane X= F, Cl, Br, I amines Alkenes Alkyl Halide Chapter 23 OH C C H O C O C C O C C Alkynes phenol alcohols ethers epoxide acidic Chapter 14 H H H C S C C C C S S C C S C C H C C sulfides thiols disulfide H H (thioethers) Arenes 253 Nomenclature of alcohols 1. In general, alcohols are named in the same manner as alkanes; replace the -ane suffix for alkanes with an -ol for alcohols CH3CH2CH2CH3 CH3CH2CH2CH2OH OH butane 1-butanol 2-butanol butan-1-ol butan-2-ol 2. Number the carbon chain so that the hydroxyl group gets the lowest number 3. Number the substituents and write the name listing the substituents in alphabetical order. Many alcohols are named using non-systematic nomenclature H C OH 3 OH OH C OH OH HO OH H3C HO H3C benzyl alcohol allyl alcohol tert-butyl alcohol ethylene glycol glycerol (phenylmethanol) (2-propen-1-ol) (2-methyl-2-propanol) (1,2-ethanediol) (1,2,3-propanetriol) 254 127 Alcohols are classified according to the H R C OH C OH H H degree of substitution of the carbon bearing H H 1° carbon the -OH group methanol primary alcohol primary (1°) : one alkyl substituent R R C OH C OH R R secondary (2°) : two alkyl substituents H R 2° carbon 3° carbon tertiary (3°) : three alkyl substituents secondary alcohol tertiary alcohol Physical properties of alcohols – the C-OH bond of alcohols has a significant dipole moment. -

A New Approach to Prepare Polyethylene Glycol Allyl Glycidyl Ether
E3S Web of Conferences 267, 02004 (2021) https://doi.org/10.1051/e3sconf/202126702004 ICESCE 2021 A new approach to prepare Polyethylene Glycol Allyl Glycidyl Ether Huizhen Wang1*, Ruiyang Xie1, Mingjun Chen1*, Weihao Deng1, Kaixin Zhang2, Jiaqin Liu1 1School of Science, Xihua University, Chengdu 610039, China; 2Chengdu Jingyiqiang Environmental Protection Technology Co., Ltd. Abstract. The polyethylene glycol allyl glycidyl ether (PGAGE) is an important intermediate for preparing silicone softener that can be synthesized from allyl alcohol polyoxyethylene ether and epichlorohydrin (ECH). The performance parameters including the concentration of ECH, initial boron trifluoride diethyl etherate (BFEE) as well as CaCl2 quality were investigated respectively. The optimum process parameters which can get high capping and low by-product rate are as follows: the ECH concentration is 2.0 M, the initial BFEE concentration is 1.65mM, and the CaCl2 dosage is 1.65g/L. Under these conditions, the maximal yield can be improved to 91.36%, the percent of capping rate is higher than 98.16%, the residual concentration of F- is only 0.63 mg/L. concentrated basic solution, in which the total yield was between 90%~91% by Matsuoka et al. [10] also use the 1 Introduction two-step reaction to synthesize AGE based on the reaction Polyethylene glycol allyl glycidyl ether (PGAGE) and the of allyl alcohol with ECH using BFEE as the catalyst. allyl polyoxyethylene ether (APEG), tethering with both Their results demonstrated that the yield reaches 82% alkene and epoxy groups, are widely used as fabric under the following condition: n (ECH) : n (allyl alcohol): finishing agent [1-2] , reactive diluent [3] , cross-linking (catalysis) = 1: (1~3) : (0.01~0.002). -

SAFETY DATA SHEET Isopropyl Alcohol
SAFETY DATA SHEET Isopropyl Alcohol Section 1. Identification GHS product identifier : Isopropyl Alcohol Chemical name : Isopropyl alcohol Other means of : isopropanol; 2-Propanol identification Product type : Liquid. Product use : Synthetic/Analytical chemistry. Synonym : isopropanol; 2-Propanol SDS # : 001105 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : FLAMMABLE LIQUIDS - Category 2 substance or mixture EYE IRRITATION - Category 2A SPECIFIC TARGET ORGAN TOXICITY (SINGLE EXPOSURE) (Narcotic effects) - Category 3 GHS label elements Hazard pictograms : Signal word : Danger Hazard statements : May form explosive mixtures with air. Highly flammable liquid and vapor. Causes serious eye irritation. May cause drowsiness or dizziness. Precautionary statements General : Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Prevention : Wear protective gloves. Wear eye or face protection. Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. Use explosion- proof electrical, ventilating, lighting and all material-handling equipment. Use only non- sparking tools. Take precautionary measures against static discharge. Keep container tightly closed. Use only outdoors or in a well-ventilated area. Avoid breathing vapor. Wash hands thoroughly after handling. Response : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Call a POISON CENTER or physician if you feel unwell. -
Title Selective Reduction of Acrolein to Allyl Alcohol by a Vapor Phase
Selective Reduction of Acrolein to Allyl Alcohol by a Vapor Title Phase Hydrogen Transfer Reaction Author(s) Kunichika, Sango; Oka, Shinzaburo; Sakai, Mutsuji Bulletin of the Institute for Chemical Research, Kyoto Citation University (1966), 44(3): 215-220 Issue Date 1966-10-31 URL http://hdl.handle.net/2433/76125 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University Selective Reduction of Acrolein to Allyl Alcohol by a Vapor Phase Hydrogen Transfer Reaction Sango KUNICHIKA,Shinzaburo OKA and Mutsuji SAKAI* (KunichikaLaboratory) (ReceivedJune 15, 1966) Selectivereduction of acroleinto ally! alcoholhas been achievedby a vapor phase hydrogen transferreaction using isopropyl alcohol as a hydrogendonor. It was foundthat the preferredcatalyst was 50 weight% zinc oxide-magnesiumoxide catalyst prepared by calcininga mixtureof zincnitrate and magnesiumoxide at 500°C. The optimumconditions were found to be as follows;reaction tem- perature,near 200°C,total feed rate, 45 moles/1.of catalyst/hr.,feed ratio of isopropylalcohol per acrolein,10 moles. Under theseconditions, the yieldsof allyl alcoholof 80-65% at 80-90% conversion of acroleinwere obtained. INTRODUCTION Acrolein has two reactive functional groups, one is carbonyl group and the other is carbon-carbon double bond. Selective reduction of acrolein to allyl alcohol has been studied by several investigators. The reaction was generally carried out by the following two methods: (1) the Meerwein-Ponndorf-Verley reduction1-3), (2) vapor phase hydrogen transfer reaction with various metal oxide catalysts using an alcohol as a hydrogen donor4-11> The liquid phase reaction has some technical difficulties owing to the polymeri- zation of acrolein especially at high conversion. Therefore, in the present work, the more promising vapor phase reaction has been studied to find an effective catalyst and favorable reaction conditions. -

Conversion of Glycerol Into Allyl Alcohol Over Potassium-Supported Zirconia-Iron Oxide Catalyst
Title Conversion of glycerol into allyl alcohol over potassium-supported zirconia-iron oxide catalyst Author(s) Konaka, Aya; Tago, Teruoki; Yoshikawa, Takuya; Nakamura, Ayaka; Masuda, Takao Applied catalysis b-environmental, 146, 267-273 Citation https://doi.org/10.1016/j.apcatb.2013.03.007 Issue Date 2014-03 Doc URL http://hdl.handle.net/2115/54715 Type article (author version) File Information Manuscript_Glycerol to Allyl Alcohol .pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP Conversion of Glycerol into Allyl Alcohol over Potassium-Supported Zirconia-Iron Oxide Catalyst Aya Konaka, Teruoki Tago*, Takuya Yoshikawa, Ayaka Nakamura, and Takao Masuda Research Group of Chemical Engineering, Division of Chemical Process Engineering, Faculty of Engineering, Hokkaido University, N13W8, Kita-ku, Sapporo, Hokkaido, Japan 060-8628 * Corresponding author should be addressed E-mail: [email protected] Tel: +81-11-706-6551 Fax: +81-11-706-6552 1 Abstract The catalytic conversion of glycerol was performed with iron oxide-based catalysts for production of allyl alcohol using a fixed-bed flow reactor at 623 K under atmospheric pressure. The glycerol dehydration proceeds on acid sites of catalysts while the allyl alcohol production is assumed to be catalyzed by non-acidic sites of catalysts through a hydrogen transfer mechanism. Different alkali metals, including Na, K, Rb, and Cs were supported on ZrO2-FeOX and all of them gave impressively higher allyl alcohol yield and suppressed glycerol dehydration due to the reduced catalyst acidic property. K-supported ZrO2-FeOX (K/ZrO2-FeOX) was chosen for further studies, and allyl alcohol yield remarkably increased up to 27 mol%-C at the K content of 3-5 mol%. -

N-BUTYL ALCOHOL
Right to Know Hazardous Substance Fact Sheet Common Name: n-BUTYL ALCOHOL Synonyms: Propyl Carbinol; n-Butanol CAS Number: 71-36-3 Chemical Name: 1-Butanol RTK Substance Number: 1330 Date: November 1998 Revision: January 2008 DOT Number: UN 1120 Description and Use EMERGENCY RESPONDERS >>>> SEE BACK PAGE n-Butyl Alcohol is a colorless liquid with a strong, sweet Hazard Summary alcohol odor. It is used as a solvent for fats, waxes, shellacs, Hazard Rating NJDOH NFPA resins, gums, and varnish, in making hydraulic fluids, and in HEALTH - 2 medications for animals. FLAMMABILITY - 3 REACTIVITY - 0 f ODOR THRESHOLD = 1 to 15 ppm FLAMMABLE f Odor thresholds vary greatly. Do not rely on odor alone to POISONOUS GASES ARE PRODUCED IN FIRE determine potentially hazardous exposures. CONTAINERS MAY EXPLODE IN FIRE Hazard Rating Key: 0=minimal; 1=slight; 2=moderate; 3=serious; Reasons for Citation 4=severe f n-Butyl Alcohol is on the Right to Know Hazardous f n-Butyl Alcohol can affect you when inhaled and by Substance List because it is cited by OSHA, ACGIH, DOT, passing through the skin. NIOSH, DEP, IRIS, NFPA and EPA. f Contact can irritate and burn the skin. f This chemical is on the Special Health Hazard Substance f n-Butyl Alcohol can irritate and burn the eyes with possible List. eye damage. f Inhaling n-Butyl Alcohol can irritate the nose, throat and lungs. f Exposure to n-Butyl Alcohol can cause headache, dizziness, nausea and vomiting. SEE GLOSSARY ON PAGE 5. f n-Butyl Alcohol can damage the liver, kidneys, hearing, and sense of balance. -
Williamson Ether Synthesis the Williamson Ether Synthesis Is an Organic Reaction, Forming an Ether from an Alkyl Halide and an Alcohol
The Williamson ether synthesis The Williamson ether synthesis is an organic reaction, forming an ether from an alkyl halide and an alcohol. This reaction was developed by Alexander Williamson in 1850. It involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction. The Williamson reaction is widely used in both laboratory and industrial synthesis, and remains the simplest and most popular method of preparing ethers. Both symmetrical and asymmetrical ethers are easily prepared. The reaction for this week: an example of a Williamson ether synthesis acetaminophen ethyl iodide phenacetin starting material reagent product Phenacetin may be synthesized as an example of the Williamson ether synthesis The first synthesis of phenacetin was reported in 1878 by Harmon Morse. Procedure 1. Weigh an Extra-Strength Tylenol tablet. Pulverize the tablet with mortar and pestle. Weigh out 0.22 g and place it in a dry 15-ml round-bottom flask along with 0.28 g of finely pulverized K2CO3 (mortar and pestle) and 3.0 mL of butanone. Carefully add 0.28 mL of ethyl iodide with a syringe. 2. Add a stir bar; attach a microscale water-cooled condenser to the flask. Heat the mixture under reflux directly on a hot plate at medium setting for 1 hour. In the meantime, obtain the IR of acetaminophen. 3. Turn off the heat. Allow the mixture to cool down. Add 4 mL of water to the flask and transfer its contents to a 16 x 125 mm test tube with a screw cap. Rinse round-bottom flask 4 times with 1 mL of tert-butyl methyl ether (BME) and add the rinsings to the test tube. -

United States Patent (19) (11) Patent Number: 4,968,451 Scheibel Et Al
United States Patent (19) (11) Patent Number: 4,968,451 Scheibel et al. 45) Date of Patent: Nov. 6, 1990 (54) SOIL RELEASE AGENTS HAVING ALLYL-DERVED SULFONATED END CAPS FOREIGN PATENT DOCUMENTS 155710 9/1985 European Pat. Off. (75) Inventors: Jeffrey J. Scheibel; Eugene P. 180356 5/1986 European Pat. Off. Gosselink, both of Cincinnati, Ohio 1475798 6/1977 United Kingdom . (73) Assignee: The Procter & Gamble Company, OTHER PUBLICATIONS Cincinnati, Ohio S. C. Bright, C. E. Stubbs and L. Thompson, J. Appl. (21) Appl. No.: 474,709 Chem. Biotechnol, 1975, vol. 25, pp. 901-912. 22) Filed: Jan. 29, 1990 Norton et al., J. Org. Chem, vol. 33, No. 11, pp. 4158-4165 (1967). Related U.S. Application Data Primary Examiner-A. Lionel Clingman (63) Continuation of Ser. No. 237,598, Aug. 26, 1988, aban Attorney, Agent, or Firm-Leonard W. Lewis; Jerry J. doned. Yetter (51 Int, C. .................... CO7C 309/05; C08G 63/68; 57 ABSTRACT C11D 3/37 (52) U.S. C. ............................... 252/549; 252/174.19; The present invention relates to novel soil release 252/539; 252/558; 252/DIG. 15 agents, which are particular sulfonated linear tere (58) Field of Search ......................................... 252/549 phthalate ester oligomers (S.T.E. oligomers). The S.T.E. oligomers are especially suitable for formulation (56 References Cited into laundry products such as laundry detergents or U.S. PATENT DOCUMENTS fabric conditioners. Thus formulated, they provide ef. 3,821,169 6/1974 Duddey et al. ..................... 528/293 fective soil release treatments for fabrics laundered in 4,156,073 5/1979 Login ............. -

How Is Alcohol Metabolized by the Body?
Overview: How Is Alcohol Metabolized by the Body? Samir Zakhari, Ph.D. Alcohol is eliminated from the body by various metabolic mechanisms. The primary enzymes involved are aldehyde dehydrogenase (ALDH), alcohol dehydrogenase (ADH), cytochrome P450 (CYP2E1), and catalase. Variations in the genes for these enzymes have been found to influence alcohol consumption, alcohol-related tissue damage, and alcohol dependence. The consequences of alcohol metabolism include oxygen deficits (i.e., hypoxia) in the liver; interaction between alcohol metabolism byproducts and other cell components, resulting in the formation of harmful compounds (i.e., adducts); formation of highly reactive oxygen-containing molecules (i.e., reactive oxygen species [ROS]) that can damage other cell components; changes in the ratio of NADH to NAD+ (i.e., the cell’s redox state); tissue damage; fetal damage; impairment of other metabolic processes; cancer; and medication interactions. Several issues related to alcohol metabolism require further research. KEY WORDS: Ethanol-to acetaldehyde metabolism; alcohol dehydrogenase (ADH); aldehyde dehydrogenase (ALDH); acetaldehyde; acetate; cytochrome P450 2E1 (CYP2E1); catalase; reactive oxygen species (ROS); blood alcohol concentration (BAC); liver; stomach; brain; fetal alcohol effects; genetics and heredity; ethnic group; hypoxia The alcohol elimination rate varies state of liver cells. Chronic alcohol con- he effects of alcohol (i.e., ethanol) widely (i.e., three-fold) among individ- sumption and alcohol metabolism are on various tissues depend on its uals and is influenced by factors such as strongly linked to several pathological concentration in the blood T chronic alcohol consumption, diet, age, consequences and tissue damage. (blood alcohol concentration [BAC]) smoking, and time of day (Bennion and Understanding the balance of alcohol’s over time. -

Phosphine-Catalyzed Additions of Nucleophiles and Electrophiles to Α
PHOSPHINE-CATALYZED ADDITIONS OF NUCLEOPHILES AND ELECTROPHILES TO α,β–UNSATURATED CARBONYL COMPOUNDS Reported by Michael Scott Bultman November 4, 2004 INTRODUCTION Organophosphorous compounds are becoming increasingly important in organic synthesis. Phosphines serve as precursors to phosphonium ylides in the Wittig reaction,1 and as nucleophilic triggers in the Mitsunobu2 and Staudinger3 reactions. In these processes, the phosphine is stoichiometrically consumed and converted into a phosphine oxide. Phosphines are also commonly used as ligands for transition metal-catalyzed reactions, to modulate reactivity and stereocontrol.4 On the other hand, the use of phosphines as nucleophilic catalysts for organic reactions has only gained attention in the last ten years. First reported by Rauhut and Currier in 1963,5 phosphine catalysis has since been reinvestigated after the phosphine ligands in some transition-metal-catalyzed reactions were found to be better catalysts than the metal/phosphine complexes alone!6 Phosphines are well suited for catalyzing the addition of both nucleophiles and electrophiles to electron deficient alkenes, alkynes, and allenes. Activation of these α,β-unsaturated carbonyl systems with the phosphine enables the formation of new bonds at the α-, β-, and γ-positions. This report will highlight these different modes of addition to α,β-unsaturated carbonyl systems under phosphine catalysis that allow for the formation of a wide array of products from a single class of substrates. GENERAL REACTIVITY OF PHOSPHINES Key characteristics required for successful nucleophilic catalysis lie in the balance of leaving group ability, nucleophilicity, and ease of ylid formation. Increasing leaving group ability can often be + correlated with decreasing basicity. -

Sugar Alcohols—Their Role in the Modern World of Sweeteners: a Review
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Springer - Publisher Connector Eur Food Res Technol (2015) 241:1–14 DOI 10.1007/s00217-015-2437-7 REVIEW PAPER Sugar alcohols—their role in the modern world of sweeteners: a review Małgorzata Grembecka Received: 31 August 2014 / Revised: 16 December 2014 / Accepted: 13 February 2015 / Published online: 28 February 2015 © The Author(s) 2015. This article is published with open access at Springerlink.com Abstract Epidemic obesity and diabetes encouraged the observed. It was mainly due to the developments in bio- changes in population lifestyle and consumers’ food prod- logical studies, the change of a population lifestyle and the ucts awareness. Food industry has responded people’s increase in the consumer awareness concerning food prod- demand by producing a number of energy-reduced prod- ucts. The health quality of food depends mainly on nutri- ucts with sugar alcohols as sweeteners. These compounds ents, but also on foreign substances such as food additives. are usually produced by a catalytic hydrogenation of carbo- The presence of foreign substances in the food can be justi- hydrates, but they can be also found in nature in fruits, veg- fied, allowed or tolerated only when they are harmless to etables or mushrooms as well as in human organism. Due our health. Epidemic obesity and diabetes encouraged the to their properties, sugar alcohols are widely used in food, growth of the artificial sweetener industry. There are more beverage, confectionery and pharmaceutical industries and more people who are trying to lose weight or keeping throughout the world.