Unit Conversion 1/2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Standard Conversion Factors
Standard conversion factors 7 1 tonne of oil equivalent (toe) = 10 kilocalories = 396.83 therms = 41.868 GJ = 11,630 kWh 1 therm = 100,000 British thermal units (Btu) The following prefixes are used for multiples of joules, watts and watt hours: kilo (k) = 1,000 or 103 mega (M) = 1,000,000 or 106 giga (G) = 1,000,000,000 or 109 tera (T) = 1,000,000,000,000 or 1012 peta (P) = 1,000,000,000,000,000 or 1015 WEIGHT 1 kilogramme (kg) = 2.2046 pounds (lb) VOLUME 1 cubic metre (cu m) = 35.31 cu ft 1 pound (lb) = 0.4536 kg 1 cubic foot (cu ft) = 0.02832 cu m 1 tonne (t) = 1,000 kg 1 litre = 0.22 Imperial = 0.9842 long ton gallon (UK gal.) = 1.102 short ton (sh tn) 1 UK gallon = 8 UK pints 1 Statute or long ton = 2,240 lb = 1.201 U.S. gallons (US gal) = 1.016 t = 4.54609 litres = 1.120 sh tn 1 barrel = 159.0 litres = 34.97 UK gal = 42 US gal LENGTH 1 mile = 1.6093 kilometres 1 kilometre (km) = 0.62137 miles TEMPERATURE 1 scale degree Celsius (C) = 1.8 scale degrees Fahrenheit (F) For conversion of temperatures: °C = 5/9 (°F - 32); °F = 9/5 °C + 32 Average conversion factors for petroleum Imperial Litres Imperial Litres gallons per gallons per per tonne tonne per tonne tonne Crude oil: Gas/diesel oil: Indigenous Gas oil 257 1,167 Imported Marine diesel oil 253 1,150 Average of refining throughput Fuel oil: Ethane All grades 222 1,021 Propane Light fuel oil: Butane 1% or less sulphur 1,071 Naphtha (l.d.f.) >1% sulphur 232 1,071 Medium fuel oil: Aviation gasoline 1% or less sulphur 237 1,079 >1% sulphur 1,028 Motor -

3 Litre User's Manual
™ 3 Litre User’s Manual Please visit www.drewandcole.com for video instructions and cooking demonstrations. 1 IMPORTANT SAFETY INFORMATION IMPORTANT SAFETY INFORMATION BEFORE YOU GET STARTED, PLEASE READ THE FOLLOWING IMPORTANT SAFETY INFORMATION, ALONG WITH THE MANUAL ENCLOSED AND KEEP PRESSURE RELEASE METHODS BOTH FOR FUTURE REFERENCE. WARNING YOU ARE WORKING WITH • When the programme is finished and you wish to commence pressure release press the “Cancel” button to cancel HOT LIQUIDS. YOU MUST READ THIS BEFORE USE. the Keep Warm function. • When releasing the pressure valve, always use tongs and please wear oven gloves to turn the pressure valve to the open position. This will protect against hot steam. The valve will lift up slightly and steam will release. The lid won’t BEFORE COOKING open until the steam has vented and pressure has released. • When opening the lid food will be hot, please always wear oven gloves and an apron to protect against any • ALWAYS ensure the INNER POT is in place before cooking. splashing of the hot food. • Food with skins (e.g sausages, chicken and fruit) MUST be pierced before cooking. Not piercing QUICK RELEASE SLOW RELEASE the skin may result in the food expanding and may cause splashing of hot food after the lid is Recommended for: Recommended for: released. - Quick cooking recipes and steaming, including - Food with skins (e.g sausages, chicken and fruit) and vegetables and seafood. foods with large liquid volume or high starch content • Do not overfill the inner pot. (such as porridge, soup, pasta, rice, fruit and grains, and When the Keep Warm function has been also delicate foods such as meats and potato) can trap air cancelled, move the pressure release valve to and cause the food to foam and expand which may cause To Max the open position and only attempt to open lid MAX MAX splashing of hot food after the lid is removed. -

Binary Operator
Table of Contents Teaching and Learning The Metric System Unit 1 1 - Suggested Teaching Sequence 1 - Objectives 1 - Rules of Notation 1 - Metric Units, Symbols, and Referents 2 - Metric Prefixes 2 - Linear Measurement Activities 3 - Area Measurement Activities 5 - Volume Measurement Activities 7 - Mass (Weight) Measurement Activities 9 - Temperature Measurement Activities 11 Unit 2 12 - Objectives 12 - Suggested Teaching Sequence 12 - Metrics in this Occupation 12 - Metric Units For Binary Operation 13 - Trying Out Metric Units 14 - Binding With Metrics 15 Unit 3 16 - Objective 16 - Suggested Teaching Sequence 16 - Metric-Metric Equivalents 16 - Changing Units at Work 18 Unit 4 19 - Objective 19 - Suggested Teaching Sequence 19 - Selecting and Using Metric Instruments, Tools and Devices 19 - Which Tools for the Job? 20 - Measuring Up in Binary Operations 20 Unit 5 21 - Objective 21 - Suggested Teaching Sequence 21 - Metric-Customary Equivalents 21 - Conversion Tables 22 - Any Way You Want It 23 Testing Metric Abilities 24 Answers to Exercises and Test 25 Tools and Devices List References TEACHING AND LEARNING THE METRIC SYSTEM Thi.s metric instructional package was designed to meet job-related Unit 2 provides the metric terms which are used in this occupation metric measurement needs of students. To use this package students and gives experience with occupational measurement tasks. should already know the occupational terminology, measurement terms, and tools currently in use. These materials were prepared with Unit 3 focuses on job-related metric equivalents and their relation the help of experienced vocational teachers, reviewed by experts, tested ships. in classrooms in different parts of the United States, and revised before distribution. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

LITRE Reports
LITRE Assessment of Ambulatory Pumps for Parenteral Nutrition Results of a LITRE Assessment Panel 29th & 30th July 2016 Published February 2017 © PINNT 2017 What is LITRE? A multi-professional panel led by patients that aims to improve the quality of life for patients on home artificial nutrition in terms of services and products. It is a standing committee of PINNT. Our Mission . Investigating and responding to the needs and concerns raised by patients, carers and healthcare professionals with regard to equipment and services. Forging links between patients, carers and industry. Acting as a forum for users to help in product and service development and market research. Representation on the Committee The LITRE committee will always be predominantly patients and carers. LITRE meets to address individual projects and the most appropriate team of experts is assembled based on the nature of each project. LITRE will invite additional experts to join the panel with the clear intention of ensuring each additional person will bring knowledge and expertise to the project in hand. Previous Projects LITRE has historically undertaken a wide ranging number of projects over the years. A full list can be found on http://pinnt.com/About-Us/LITRE.aspx LITRE Panel Members PINNT advertised the proposed LITRE assessment in Online, on Facebook and on the website. Clear information was given in terms of how the assessment would work as the previous format has been revised. All applicants were asked to complete a simple questionnaire (Appendix 1). We were seeking applicants to represent the diverse feeding regimes as well as the various lifestyles in which home parenteral feeding is used and administered. -

Introducyon to the Metric System Bemeasurement, Provips0.Nformal, 'Hands-On Experiences For.The Stud4tts
'DOCUMENT RESUME ED 13A 755 084 CE 009 744 AUTHOR Cáoper, Gloria S., Ed.; Mag4,sos Joel B., Ed. TITLE q Met,rits for.Theatrical COstum g: ° INSTITUTION Ohio State Univ., Columbus. enter for Vocational Education. SPONS AGENCY Ohio State Univ., Columbus. Center for.Vocational Education. PUB DATE 76 4. CONTRACT - OEC-0-74-9335 NOTE 59p.1 For a. related docuMent see CE 009 736-790 EDRS PRICE 10-$0.8.3 C-$3.50 Plus Pdstage: DESCRIPTORS *Curriculu; Fine Arts; Instructional Materials; Learning. ctivities;xMeasurement.Instrnments; *Metric, System; S condary Education; Teaching Technigue8; *Theater AttS; Units of Sttidy (Subject Fields) ; , *Vocational Eiducation- IDENTIFIERS Costumes (Theatrical) AESTRACT . Desigliedto meet tbe job-related m4triceasgrement needs of theatrical costuming students,'thiS instructio11 alpickage is one of live-for the arts and-huminities occupations cluster, part of aset b*: 55 packages for:metric instrection in diftepent occupations.. .The package is in'tended for students who already knovthe occupaiiOnal terminology, measurement terms, and tools currently in use. Each of the five units in this instructional package.contains performance' objectiveS, learning:Activities, and'supporting information in-the form of text,.exertises,- ard tabled. In. add±tion, Suggested teaching technigueS are included. At the'back of the package*are objective-base'd:e"luation items, a-page of answers to' the exercises,and tests, a list of metric materials ,needed for the ,activities4 references,- and a/list of supPliers.t The_material is Y- designed. to accVmodate awariety of.individual teacting,:and learning k. styles, e.g., in,dependent:study, small group, or whole-class Setivity. -

Calculating Spray Application Information
Calculating Spray Application Information Knowing how your sprayer is calibrated is critical if you want to determine spray application information for a specific pesticide in a sp ecific field. T his factsheet will deal with the math required to determine specific spray application information. In addition to sprayer output (L/ha or gal/acre) it is critical to know the area of the field you wish to spray, the amount of product required per unit area (kg/ha or L/ha) and the water capacity of your sprayer. By knowing these variables the amount of product that needs to be added to a tank full of water and the number of tank loads that will be applied to that field can be determined. This can be critical, as it allows the applicator to mix only the amount of product needed, saving dollars and the environment. Many producers like to work in imperial units. This can create challenges as all pesticide labels are written in metric units. T his exam includes a conversion sheet which will help producers work with the units they are most comfortable with. That being said, if you are working in metric or imperial units the principles for determining spray application information is the same. Caution: When converting from imperial to metric or vice-versa, be very careful when rounding numbers as errors can cause changes to application rates and lead to either loss of pest control or potential crop damage. When a sprayer is calculated we know the sprayer output (Volume/Area; ie. 200 L/ha) and the spray tank capacity (Volume; ie. -

The International System of Units (SI)
The International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 330 2008 Edition Barry N. Taylor and Ambler Thompson, Editors NIST SPECIAL PUBLICATION 330 2008 EDITION THE INTERNATIONAL SYSTEM OF UNITS (SI) Editors: Barry N. Taylor Physics Laboratory Ambler Thompson Technology Services National Institute of Standards and Technology Gaithersburg, MD 20899 United States version of the English text of the eighth edition (2006) of the International Bureau of Weights and Measures publication Le Système International d’ Unités (SI) (Supersedes NIST Special Publication 330, 2001 Edition) Issued March 2008 U.S. DEPARTMENT OF COMMERCE, Carlos M. Gutierrez, Secretary NATIONAL INSTITUTE OF STANDARDS AND TECHNOLOGY, James Turner, Acting Director National Institute of Standards and Technology Special Publication 330, 2008 Edition Natl. Inst. Stand. Technol. Spec. Pub. 330, 2008 Ed., 96 pages (March 2008) CODEN: NSPUE2 WASHINGTON 2008 Foreword The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant system used in science, the SI is rapidly becoming the dominant measurement system used in international commerce. In recognition of this fact and the increasing global nature of the marketplace, the Omnibus Trade and Competitiveness Act of 1988, which changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. industry increase its competitiveness, designates “the metric system of measurement as the preferred system of weights and measures for United States trade and commerce.” The definitive international reference on the SI is a booklet published by the International Bureau of Weights and Measures (BIPM, Bureau International des Poids et Mesures) and often referred to as the BIPM SI Brochure. -
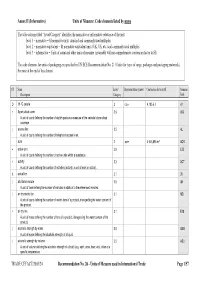
Units of Measure Used in International Trade Page 1/57 Annex II (Informative) Units of Measure: Code Elements Listed by Name
Annex II (Informative) Units of Measure: Code elements listed by name The table column titled “Level/Category” identifies the normative or informative relevance of the unit: level 1 – normative = SI normative units, standard and commonly used multiples level 2 – normative equivalent = SI normative equivalent units (UK, US, etc.) and commonly used multiples level 3 – informative = Units of count and other units of measure (invariably with no comprehensive conversion factor to SI) The code elements for units of packaging are specified in UN/ECE Recommendation No. 21 (Codes for types of cargo, packages and packaging materials). See note at the end of this Annex). ST Name Level/ Representation symbol Conversion factor to SI Common Description Category Code D 15 °C calorie 2 cal₁₅ 4,185 5 J A1 + 8-part cloud cover 3.9 A59 A unit of count defining the number of eighth-parts as a measure of the celestial dome cloud coverage. | access line 3.5 AL A unit of count defining the number of telephone access lines. acre 2 acre 4 046,856 m² ACR + active unit 3.9 E25 A unit of count defining the number of active units within a substance. + activity 3.2 ACT A unit of count defining the number of activities (activity: a unit of work or action). X actual ton 3.1 26 | additional minute 3.5 AH A unit of time defining the number of minutes in addition to the referenced minutes. | air dry metric ton 3.1 MD A unit of count defining the number of metric tons of a product, disregarding the water content of the product. -

United States Patent ICC Patented Feb
,. 3,716,412 United States Patent ICC Patented Feb. 13, 1973 1 2 A general description of the nature and properties of 3,716,412 ' ELECTRIC STORAGE BATTERIES amorphous silicas useful in the present invention is con Kenneth Peters, Walkden, England, assignor to Electric tained in the article by S. A. Mitchell at pages 924 to Power Storage Limited, Swinton, Manchester, England 933 of “Chemistry and Industry,” 1966. ' ' No Drawing. Filed Feb. 19, 1971, Ser. No. 117,119 The disclosures of these two documents are incorpo Int. Cl. H01m 9/00, 35/30 rated herein by reference. ‘ US. Cl. 136—34 6 Claims The amorphous silicagcan instead be formed in situ from a silicate such as sodium silicate which does not possess as intefering ion. Thus the amorphous silica can ABSTRACT OF THE DISCLOSURE be produced merely by adding sodium silicate to the acid A lead acid battery having an improved acid electrolyte 10 electrolyte in an amount such that the electrolyte in use is disclosed. The electrolyte in the battery contains not contains up to 75 grams per litre of amorphous silica. more than 75 grams of amorphous silica (calculated as The electrolyte may be that which will remain in the SiO2) per litre of electrolyte whereby the resistance to battery throughout its life or can be that which is used shedding of active material from the positive electrode 15 merely for the electrolytic formation of the green pasted of the battery is enhanced. ' positive plates. The plates can then be used either with the novel electrolyte or a vconventional electrolyte in the battery. -

Siunitx – a Comprehensive (Si) Units Package∗
siunitx – A comprehensive (si) units package∗ Joseph Wright† Released 2021-09-22 Contents 1 Introduction 3 2 siunitx for the impatient 3 3 Using the siunitx package 4 3.1 Numbers . 4 3.2 Angles . 5 3.3 Units . 6 3.4 Complex numbers and quantities . 7 3.5 The unit macros . 8 3.6 Unit abbreviations . 10 3.7 Creating new macros . 14 3.8 Tabular material . 15 4 Package control options 17 4.1 The key–value control system . 17 4.2 Printing . 18 4.3 Parsing numbers . 20 4.4 Post-processing numbers . 22 4.5 Printing numbers . 25 4.6 Lists, products and ranges . 28 4.7 Complex numbers . 31 4.8 Angles . 32 4.9 Creating units . 34 4.10 Using units . 35 4.11 Quantities . 38 4.12 Tabular material . 39 4.13 Locale options . 49 4.14 Preamble-only options . 49 5 Upgrading from version 2 49 6 Unit changes made by BIPM 51 ∗This file describes v3.0.31, last revised 2021-09-22. †E-mail: [email protected] 1 7 Localisation 52 8 Compatibility with other packages 52 9 Hints for using siunitx 52 9.1 Adjusting \litre and \liter ........................ 52 9.2 Ensuring text or math output . 53 9.3 Expanding content in tables . 53 9.4 Using siunitx with datatool .......................... 54 9.5 Using units in section headings and bookmarks . 55 9.6 A left-aligned column visually centred under a heading . 56 9.7 Regression tables . 57 9.8 Maximising performance . 58 9.9 Special considerations for the \kWh unit . -

Capacity and Volume
Capacity and Volume Capacity Capacity is the amount a container can hold. The oil, juice drink and gasoline containers are just a few examples of objects that illustrate capacity. Capacity is measured in the SI base unit called litres (L). The most common units for capacity are litre (L) and millilitre (mL). Hint: Remember to use this 1000 L kilolitre (kL) ACRONYM to help you with the order 100 g hectolitre (hL) of the units: 10 g decalitre (daL) King 1 g litre (L) Henry’s 1/10 g decilitre (dL) Daughter 1/100 g centilitre (cL) Betty 1/1000g millilitre (mL) Detested Counting Money Knowledge and Employability Studio Mathematics Shape and Space: Measurement: ©Alberta Education, Alberta, Canada (www.LearnAlberta.ca) Capacity and Volume 1/6 Recipes require ingredients in specific amounts to create the desired finished product. Not all ingredients come packaged in the quantities that recipes call for, so converting between different units of capacity is important. Examples A) How many mL does 10 L represent? 10 000 mL B) How many L does 4000 mL represent? 4 L C) How many mL does 7.4 L represent? 7400 mL Volume Volume is the amount of space a container or object occupies. Capacity is the amount of water required to Example fill the fish tank (ml or L). Volume is the space the tank and water take up. The most common unit of volume is centimetres cubed (cm3). One centimetre cubed will hold one millilitre of fluid or another substance. 1000 cm3 will hold one thousand millilitres of fluid or another substance.