Habitat Use and Population Viability of the Red Kite (Milvus Milvus) in Germany
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Heilige Messen Im Pastoralverbund „Maria Hilf – Schwalmstadt“, Dekanat Fritzlar, Bistum Fulda
Heilige Messen im Pastoralverbund „Maria Hilf – Schwalmstadt“, Dekanat Fritzlar, Bistum Fulda Homepage: www.pastoralverbund-schwalmstadt.de Monatliche Pfarrmitteilung der Kirchengemeinden (Gottesdienste u.a.) unter Rubrik „Aktuelles“ Auskunft gibt: Zentrale Pfarrbüro Ziegenhain 06691 3227 [email protected] Herzliche Einladung zu unseren Gottesdiensten, Heiligen Messen und Veranstaltungen! Auf der Homepage finden Sie unter der Rubrik „Aktuelles“, die monatlichen Pfarrmitteilungen der Kirchengemeinden. Änderungen werden i.d.R. in den monatlichen Pfarrmitteilungen mitgeteilt. Kirchen der Kirchengemeinden St. Josef und Maria Hilf: Kirchengemeinde St. Josef Schwalmstadt-Neukirchen (Ziegenhain, Frielendorf, Neukirchen, Oberaula, Schrecksbach und die Ortschaften) und Kirchengemeinde Maria-Hilf Trutzhain Pfarrbüro in Ziegenhain: 06691 3227 [email protected] Pfarrer Jens Körber Gemeindereferentin Mechthild Mühle Abwechselnd in den Kirchen findet einmal im Monat eine Wortgottesfeier statt. Info steht in der monatlichen Pfarrmitteilung. Maria Hilf Kirche in Trutzhain, Am Spielplatz 3 samstags um 18 Uhr St. Johannes d.T. Kirche in Frielendorf, Homberger Str. 3 14-tägig samstags um 17 Uhr (siehe monatliche Pfarrmitteilung) Steinweg 51, 34613 Schwalmstadt; Telefon (06691) 3227, Pfarrer: (06691) 220 4676 [email protected]; www.pastoralverbund-schwalmstadt.de St. Adalbert Kirche in Neukirchen, Siebenbürgenerweg 7 sonntags um 9 Uhr St. Josef Kirche in Ziegenhain, -
All Clubs Missing Officers 2014-15.Pdf
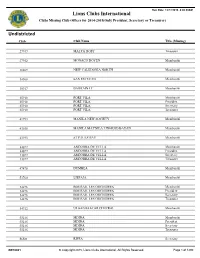
Run Date: 12/17/2015 8:40:39AM Lions Clubs International Clubs Missing Club Officer for 2014-2015(Only President, Secretary or Treasurer) Undistricted Club Club Name Title (Missing) 27947 MALTA HOST Treasurer 27952 MONACO DOYEN Membershi 30809 NEW CALEDONIA NORTH Membershi 34968 SAN ESTEVAN Membershi 35917 BAHRAIN LC Membershi 35918 PORT VILA Membershi 35918 PORT VILA President 35918 PORT VILA Secretary 35918 PORT VILA Treasurer 41793 MANILA NEW SOCIETY Membershi 43038 MANILA MAYNILA LINGKOD BAYAN Membershi 43193 ST PAULS BAY Membershi 44697 ANDORRA DE VELLA Membershi 44697 ANDORRA DE VELLA President 44697 ANDORRA DE VELLA Secretary 44697 ANDORRA DE VELLA Treasurer 47478 DUMBEA Membershi 53760 LIEPAJA Membershi 54276 BOURAIL LES ORCHIDEES Membershi 54276 BOURAIL LES ORCHIDEES President 54276 BOURAIL LES ORCHIDEES Secretary 54276 BOURAIL LES ORCHIDEES Treasurer 54912 ULAANBAATAR CENTRAL Membershi 55216 MDINA Membershi 55216 MDINA President 55216 MDINA Secretary 55216 MDINA Treasurer 56581 RIFFA Secretary OFF0021 © Copyright 2015, Lions Clubs International, All Rights Reserved. Page 1 of 1290 Run Date: 12/17/2015 8:40:39AM Lions Clubs International Clubs Missing Club Officer for 2014-2015(Only President, Secretary or Treasurer) Undistricted Club Club Name Title (Missing) 57293 RIGA RIGAS LIEPA Membershi 57293 RIGA RIGAS LIEPA President 57293 RIGA RIGAS LIEPA Secretary 57293 RIGA RIGAS LIEPA Treasurer 57378 MINSK CENTRAL Membershi 57378 MINSK CENTRAL President 57378 MINSK CENTRAL Secretary 57378 MINSK CENTRAL Treasurer 59850 DONETSK UNIVERSAL -

Standort Schwalmstadt-Ziegenhain
Standort Schwalmstadt-Ziegenhain Empfang / Anmeldung / Neukunden 06691 / 8068-101 Telefax: 06691 / 8068-120 Leistung Markt & Integration Anrede Name Vorwahl Durchwahl Anrede Name Vorwahl Durchwahl Herr Bieber 06691 8068-104 Frau Becker 06691 8068-125 Frau Enis 06691 8068-208 Frau Bromm 06691 8068-114 Frau Faust 06691 8068-203 Frau Gabel 06691 8068-122 Herr Müller 06691 8068-209 Frau Kappel 06691 8068-119 Frau Nebe 06691 8068-218 Frau Fiebig 06691 8068-121 Frau Gärtner 06691 8068-102 Herr Köhler 06691 8068-118 Herr Reuber 06691 8068-210 Frau Wendel 06691 8068-113 Frau Riebeling 06691 8068-223 Frau Lehsau 06691 8068-124 Frau Schlitt 06691 8068-206 Frau Pinkpank 06691 8068-116 Frau Schubert 06691 8068-205 Herr Senftleben 06691 8068-117 Frau Weiss 06691 8068-211 Frau Stein 06691 8068-123 Frau Wolter 06691 8068-227 Herr Trebes 06691 8068-115 Standort Melsungen Empfang / Anmeldung / Neukunden 05661 / 9291-10 Telefax: 05661 / 9291-61 Leistung Markt & Integration Anrede Name Vorwahl Durchwahl Anrede Name Vorwahl Durchwahl Frau Balk 05661 9291-50 Frau Bettenhausen 05661 9291-13 Frau Frese 05661 9291-19 Frau Grasso 05661 9291-48 Frau Freund 05661 9291-38 Herr Klagge 05661 9291-17 Frau Holl 05661 9291-37 Herr Kremer 05661 9291-14 Frau Jungmann 05661 9291-46 Herr Lange 05661 9291-20 Frau Köbberling 05661 9291-42 Frau Pelikan 05661 9291-53 Frau Strozda 05661 9291-33 Frau Plenker 05661 9291-16 Frau Prien 05661 9291-52 Frau Schaub 05661 9291-12 Herr Eckhardt 05661 9291-40 Frau Schweitzer 05661 9291-18 Herr Hinrichs 05661 9291-34 Herr Sengler 05681 9367-81 -

Willingshausen Knüllwald Homberg (Efze) Neustadt Schwalmstadt-Treysa Borken (Hessen) Oberaula
Kl Knüllwald KULTURSOMMER NORDHESSEN PRÄSENTIERT: „Das Flüstern der Bäume“ -Sa 6. Juli 2019, 17.00 Uhr Achim von Armin liebte das Bad im Walde, Odysseus baute sich ein Bett im Baum, der amerikanische Biologe David Haskell sam - melte Erkenntnisse über einen Quadratmeter Waldboden im All - gemeinen und Heinz Ehrhardt über die Made im Besonderen. Stefan Becker nimmt die Besucher mit auf eine erkenntnisreiche Exkursion in den Wald. Waldlesung – Sitzgelegenheit bitte selbst mitbringen. Festes Schuhwerk wird empfohlen. Familienpro - gramm im Rahmen der Veranstaltungsreihe I-Kultur. Weitere Infos: https://www.kultursommer-nordhessen.de/ de/veranstal - tungen/detail/das-flustern-der-baume/105. Ort: Burgruine Wal - lenstein,34593 Knüllwald - Wallenstein. Neustadt Teichfest in Speckswinkel Fr 5. - So 7. Juli 2019 Vereine aus Speckswinkel laden ein zum Teichfest. Ort: Dorfteich Speckswinkel, 35279 Neustadt-Speckswinkel Oberaula Foto: di Sommermarkt in Oberaula mit Märchenwelt rund um die Kir- che - Sa 6. Juli 2019, 20.00 Uhr - Radspaßveranstaltung "Die Ottertaler" eröffnen um 20.00 Uhr den Markt mit Musik und Tanz. Grußwort vom Bürgermeister Klaus Wagner und dem Borken (Hessen) Marktleiter. An diesem Abend werden Sie mit Getränken und Sonderausstellung im Themenpark Kohle & Energie „15 Jahre Speisen verwöhnt. Ort: Marktplatz Oberaula, Marktstraße 13. Themenpark – Ein Dankeschön“ Do 04. Juli - 25. Oktober 2019 Die Sonderausstellung ist bis zum 25. Oktober zu den üblichen Öffnungszeiten des Museums zu besichtigen. Gruppen können Schwalmstadt-Treysa unter 05682 808-271 oder per Mail bergbaumuseum@borken- hessen.de individuelle Führungszeiten vereinbaren. Die Sonder - Schwälmer Sommer Sause ausstellung zeigt das außergewöhnliche Engagement und Fr 5. - So 7. Juli 2019 welche Arbeiten für den Freilichtbereich in den Jahren notwen - Die Schwälmer Burschen laden zur Schwälmer Sommer Sause dig waren. -

Wss-2017-03-Jva.Pdf
Themenkarten - Wussten Sie schon, ...? - JVA (Justizvollzugsanstalten, über 250 Plätze) und JSA (Jugendstrafanstalten) - Kiel Schleswig- Holstein Neumünster Waldeck Lübeck Bützow Fuhlsbüttel Mecklenburg-Vorpommern Bremervörde Billweder Hamburg Oldenburg Bremen Bremen Neunkirchen - ASt. Neuruppin-Wulkow Meppen Niedersachsen Tegel Plötzensee Lingen - Abt. Groß Hespe Berlin Hannover Berlin Moabit Brandenburg an der Havel Sehnde Berlin-Heidering Burg Herford Bielefeld- Wolfenbüttel Brandenburg Münster Brackwede Luckau-Duben Bielefeld-Senne Rosdorf - Wolfenbüttel - Goslar Abt. Einbeck Bochum Castrop- Nordrhein- Sachsen-Anhalt Gelsen- Rauxel Cottbus-Disschen Geldern kirchen Dortmund Rosdorf - Westfalen Rosdorf Abt. Duderstadt Halle - Nbst. Essen Werl Torgau Moers-Kapellen Iserlohn Halle W.-Ronsdorf Kassel Leipzig Willich Schwerte Bautzen Düsseldorf Hagen Zeithain Remscheid Dresden Wuppertal- Tonna Vohwinkel Remscheid - ZWA Regis-Breitlingen Heinsberg Köln Schwalmstadt Thüringen Sachsen Aachen Siegburg Arnstadt Hohenleuben Zwickau Hünfeld Rheinbach Suhl-Goldlauter Euskirchen Hessen Butzbach Untermaßfeld Diez Rheinland-Pfalz Frankfurt am Main Wiesbaden Justizvollzugsanstalt Wittlich St. Georgen-Bayreuth Dieburg Jugendstrafanstalt Wittlich Rohrbach Ebrach Würzburg Weiterstadt Frankenthal Nürnberg Amberg Adelsheim Saarland Schifferstadt Mannheim Saarbrücken Zweibrücken Bruchsal Heilbronn Bayern Schwäbisch Hall Straubing Heimsheim Stuttgart Kaisheim Niederschönenfeld Baden-Württemberg Landshut Offenburg Aichach Augsburg-Gablingen Offenburg - ASt. -

Bahnradwegrotkäppchenland • Bürgerpark Bahnhofstraße 38, Tel.: 06691 918807 Schwalmstadt • Gasthaus „Am Brauhaus“, Ascherröder Str
ANZEIGEN ANZEIGEN SEHENSWÜRDIGKEITEN GASTRONOMIE Neustadt (Hessen) • Junker-Hansen-Turm Schwalmstadt-Treysa • Historisches Rathaus • Schwälmer Brotladen/Café BahnradwegRotkäppchenland • Bürgerpark Bahnhofstraße 38, Tel.: 06691 918807 Schwalmstadt • Gasthaus „Am Brauhaus“, Ascherröder Str. 1, Tel.: 06691 1559 • Totenkirche, Schwalmstadt-Treysa Schwalmstadt-Ziegenhain In Schwalmstadt, Neukirchen & Gilserberg. • Marktplatz, Johannisbrunnen, Märchenfiguren, Schwalmstadt-Treysa Wir freuen uns auf Ihren Besuch • Museum der Schwalm, Schwalmstadt-Ziegenhain • Hotel/ Restaurant Rosengarten, Muhlystr. 3, Tel.: 06691 94700 in unseren Brotläden und Cafés! • Wasserfestung Ziegenhain, Schwalmstadt-Ziegenhain • Hotel/ Restaurant Landgraf, • Tourist-Info, Schwalmstadt-Ziegenhain Landgraf-Philipp-Str. 3, Tel.: 06691 4083 Bahnradweg • Restaurant „Zum Schwälmer“, Neukirchen Wiederholdstr. 4, Tel.: 06691 3881 • Märchenhaus • Schwälmer Brotladen/Café, Rotkäppchenland WIR • Türmerstube Landgraf-Philipp-Straße 9, Tel.: 06691 9215542 sind • Heimatmuseum • La Copa, Weinstube und Café, Paradeplatz 2, Tel.: 06691 9270164 • Tourist-Info REGIONal • Hotel/Restaurant Hof Weidelbach, Ottrau-Schorbach Am Weidelbach, Tel. 06691 4726 Einfach • Steinmühle mit Mühlenmuseum Neukirchen Märchenhaft Oberaula • Landhotel Combecher/ Biergarten, Bahnhofstraße 28 • Treysa • Golfplatz Kurhessenstr. 32, Tel.: 06694 9780 • Waldschwimmbad Radeln. Tel.: 06691 24274 • Schwälmer Brotladen/ Café, Kurhessenstr. 33, Tel.: 06694 202 • Marktplatz mit Kirche • Wirtshaus zum hungriger Wolf, Stadtpark -

Volunteer Translator Pack
TRANSLATION EDITORIAL PRINCIPLES 1. Principles for text, images and audio (a) General principles • Retain the intention, style and distinctive features of the source. • Retain source language names of people, places and organisations; add translations of the latter. • Maintain the characteristics of the source even if these seem difficult or unusual. • Where in doubt make footnotes indicating changes, decisions and queries. • Avoid modern or slang phrases that might be seem anachronistic, with preference for less time-bound figures of speech. • Try to identify and inform The Wiener Library about anything contentious that might be libellous or defamatory. • The Wiener Library is the final arbiter in any disputes of style, translation, usage or presentation. • If the item is a handwritten document, please provide a transcription of the source language as well as a translation into the target language. (a) Text • Use English according to the agreed house style: which is appropriate to its subject matter and as free as possible of redundant or superfluous words, misleading analogies or metaphor and repetitious vocabulary. • Wherever possible use preferred terminology from the Library’s Keyword thesaurus. The Subject and Geographical Keyword thesaurus can be found in this pack. The Institutional thesaurus and Personal Name thesaurus can be provided on request. • Restrict small changes or substitutions to those that help to render the source faithfully in the target language. • Attempt to translate idiomatic expressions so as to retain the colour and intention of the source culture. If this is impossible retain the expression and add translations in a footnote. • Wherever possible do not alter the text structure or sequence. -

Wie Funktioniert Kommunalpolitik in Felsberg?
Stadtverband Felsberg Auf uns ist Verlass! Gestern – Heute – Morgen SPD Wie funktioniert Kommunalpolitik in Felsberg? Vorsitzender Stadtverband Felsberg Andreas Hahn Fraktionsvorsitzender Stephan Bürger Erstellt: Sebastian Obst Inhalt der Präsentation 1. Das politische Leben in Deutschland 2. Gewaltenteilung und Staatsaufbau 3. Das politische Leben in Felsberg 3.1 Der Bürgermeister 3.2 Die Stadtverordnetenversammlung 3.3 Der Magistrat 3.4 Die Ausschüsse 3.5 Die Ortsbeiräte, Ortsvorsteherinnen und Ortsvorsteher 4. Möglichkeiten der Mitwirkung, Mitbestimmung und Teilhabe für Bürgerinnen und Bürger 5. Organisationsstruktur 6. Der SPD-Unterkreis Schwalm-Eder-Nord 7. Der SPD-Stadtverband Felsberg 8. Die SPD-Ortsvereine 9. Der Kreistag Erstellt: Sebastian Obst Vorbemerkungen Diese Präsentation ist mit dem Ziel erstellt worden, den Bürgerinnen und Bürgern der Stadt Felsberg und ihren Ortsbezirken die Kommunalpolitik in der Stadt und den Ortsbezirken näherzubringen und Transparenz zu erzeugen. Die komplexen Zusammenhänge zu durchblicken ist nicht immer einfach. Uns ist es wichtig, klar zu erläutern, wie und warum etwas funktioniert oder wer wofür zuständig ist. Mit dieser Präsentation wollen wir Sie als Bürgerin oder Bürger unterstützen, sich einzubringen und das Leben in Felsberg mitzugestalten. Mehr Transparenz und besseres Verständnis macht das Mitreden und Mitmachen leichter! Egal ob Sie sich nur informieren wollen oder Interesse haben, ehrenamtlich mitzuarbeiten: Wir stehen Ihnen für alle Fragen sehr gerne zur Verfügung! Ihre Meinung ist uns wichtig! Andreas Hahn Vorsitzender Stadtverband Felsberg Erstellt: Sebastian Obst 1. Das politische Leben in Deutschland Das politische Leben in Deutschland ist mehr als Kanzleramt, Bundes- oder Landesregierungen, Bundestag oder Bundesparteien. Politik ist nicht gottgegeben oder ein Naturgesetz: Überall sind es ganz normale Menschen, die sich für das Gemeinwohl einsetzen und damit unsere Demokratie mit Leben füllen. -

Gilserberg Schwalmstadt-Treysa
Montag - Freitag Verkehrsbeschränkungen S S Anmerkungen O 99 O O O O O O O Gilserberg-Lischeid ab 6.10 8.10 10.10 12.10 14.10 16.10 18.10 20.10 Gilserberg-Heimbach 6.11 8.11 10.11 12.11 14.11 16.11 18.11 20.11 Gilserberg-Winterscheid 6.13 8.13 10.13 12.13 14.13 16.13 18.13 20.13 461 Gilserberg-Itzenhain 6.16 8.16 10.16 12.16 14.16 16.16 18.16 20.16 Bellnhausen 6.17 8.17 10.17 12.17 14.17 16.17 18.17 20.17 Gilserberg-Appenhain 6.18 8.18 10.18 12.18 14.18 16.18 18.18 20.18 b Gilserberg-Sachsenhausen, Im Bruch 6.20 8.20 10.20 12.20 14.20 16.20 18.20 20.20 0 Neue Straße 6.21 8.21 10.21 12.21 14.21 16.21 18.21 20.21 Gilsberg-Sebbeterode 6.23 6.31 8.23 10.23 12.23 14.23 16.23 18.23 20.23 Gilsberg-Schönau 6.25 6.27 8.25 10.25 12.25 14.25 16.25 18.25 20.25 Gilsberg-Schönstein 6.26 6.21 8.26 10.26 12.26 14.26 16.26 18.26 20.26 Gilserberg-Moischeid 6.28 6.30 - 8.28 10.28 12.28 14.28 16.28 18.28 20.28 Berghecke 6.29 6.31 - 8.29 10.29 12.29 14.29 16.29 18.29 20.29 Gilserberg, Post - 6.32 - - - - - - - - Raiffeisen 6.30 6.34 - 8.30 10.30 12.30 14.30 16.30 18.30 20.30 Gilserberg-Sachsenhausen, Neue Straße 6.32 - - 8.32 10.32 12.32 14.32 16.32 18.32 20.32 Schwalmstadt-Frankenhain 6.33 - 6.45 8.33 10.33 12.33 14.33 16.33 18.33 20.33 Schwalmstadt-Florshain 6.35 - - 8.35 10.35 12.35 14.35 16.35 18.35 20.35 Schwalmstadt-Treysa, Gesundheitszentrum 6.36 - - 8.36 10.36 12.36 14.36 16.36 18.36 20.36 Hephata 6.37 6.44 6.51 8.37 10.37 12.37 14.37 16.37 18.37 20.37 Bahnhof 6.40 6.47 - 8.40 10.40 12.40 14.40 16.40 18.40 20.40 Walkmühlenweg - 6.54 Wiegelsweg - 6.55 Europabad - 6.57 Brauerei 6.50 7.01 Fa Freudenberg 6.52 7.03 Ostergrundschule - 7.04 Schwalmstadt-Ascherode, Am Brunnenplatz 6.54 7.07 Ascherode 6.55 7.08 Schwalmstadt-Ziegenhain, Schuh-Rohde 6.56 7.09 Südbahnhof 6.57 7.10 Museum 6.58 7.11 Schwalmstadt-Treysa Alleeplatz 6.59 7.12 Wiederholdstr A 7.01 7.14 Kasseler Straße - - C-B-Schule an 7.07 7.20 Knieling Touristik S an Schultagen O AnrufSammelTaxi (AST): 99 fährt Haltestellen in anderer Reihenfolge an Anmeldung bis 30 Min. -

Spendenbereich, Schwalmstadt-Treysa
Hephata Hessisches Diakoniezentrum e. V., Schwalmstadt-Treysa - Spendenbereich, Schwalmstadt-Treysa - Bericht über die Prüfung des Jahresabschlusses zum 31. Dezember 2012 unverbindliches Ansichtsexemplar des endgültigen Berichts unverbindliches Ansichtsexemplar des endgültigen Berichts Inhaltsverzeichnis Seite Abkürzungsverzeichnis A. Prüfungsauftrag 1 B. Gegenstand, Art und Umfang der Prüfung 2 C. Feststellungen und Erläuterungen zur Rechnungslegung 4 I. Ordnungsmäßigkeit der Rechnungslegung 4 1. Vorjahresabschluss 4 2. Buchführung und weitere geprüfte Unterlagen 4 3. Jahresabschluss 5 II. Gesamtaussage des Jahresabschlusses 6 D. Feststellungen aus Erweiterungen des Prüfungsauftrags 7 E. Wiedergabe des Bestätigungsvermerks und Schlussbemerkung 10 Anlagen Inhaltsverzeichnis Jahresabschluss Blatt Bilanz zum 31. Dezember 2012 Gewinn- und Verlustrechnung für die Zeit vom 1. Januar bis 31. Dezember 2012 Bestätigungsvermerk des Abschlussprüfers Rechtliche Verhältnisse 1 Fragenkatalog zur erweiterten Prüfung und Berichterstattung über die Grundsätze des Deutschen Spendenrates e. V., Berlin 4 Allgemeine Auftragsbedingungen für Wirtschaftsprüfer und Wirtschaftsprüfungsgesellschaften vom 1. Januar 2002 Hinweis: Aus rechentechnischen Gründen können in Tabellen und bei Verweisen Rundungs- differenzen zu den sich mathematisch exakt ergebenden Werten (Geldeinheiten, Prozentangaben usw.) auftreten. 23749.07 2012 unverbindliches Ansichtsexemplar des endgültigen Berichts Abkürzungsverzeichnis AVR-KW Arbeitsvertragslinien für den Bereich des Diakonischen -

Carsharing Für Schwalmstadt
Unser Angebot Wie kann ich daran CarSharing teilnehmen? Der diakonische Verein „Altenhilfe Treysa e.V.“ stellt einen neu für • Einmalig anmelden unter angeschafften PKW seinen rund 06691 / 22 888 bei der Altenhilfe 300 Mitgliedern und auch Schwalmstadt Treysa Nichtmitgliedern zur Verfügung und • Bei einem Ortstermin in der bietet an, ein Auto zu teilen. von der Geschäftsstelle der Altenhilfe einen Chip (Folie) zum Öffnen des Damit können Sie unabhängiger Altenhilfe Treysa Fahrzeugs auf den Führerschein werden vom eigenen PKW und kleben lassen in Zusammenarbeit mit Gemeinschaftsfahrten sind leichter Regio.Mobil möglich. Das schont die Umwelt und entlastet Sie von der Wie funktioniert das? Anschaffung und vom Unterhalt eines eigenen PKW. • Online das Fahrzeug reservieren • Mit dem Chip auf dem Führer- Bei dem PKW handelt es sich um schein das Fahrzeug öffnen einen größeren, aber noch • Den Schlüssel aus dem Hand- handlichen Mittelklassewagen mit schuhfach herausnehmen und insgesamt sieben Sitzen. Der losfahren Wagen steht am Rande der • Tanken mit der Tankkarte im Altstadt von Treysa bereit und kann Fahrzeug verlässlich über das Internet • Bei jeglichen Problemen die Hotline reserviert werden. anrufen unter 06691 / 832 99 71 • Nach der Fahrt das Fahrzeug wieder an der Station abstellen und mit dem Führerschein verschließen Altenhilfe Treysa e.V. Wie viel kostet es? Das erste CarSharing-Auto Der Verein Altenhilfe Treysa berät seit Kilometerpreis: über 35 Jahren alte Menschen, die • bis 100 km: 0,27 € Hilfen im Alltag benötigen, und • ab 101. km: 0,10 € (pro Tag) organisiert begleitete Fahrten zum Arzt oder zum Einkauf. Dieses geschieht mit Stundenpreis: drei kleinen Fahrzeugen. Der Verein • 07:00 - 22:00 Uhr: 2,00 € betreibt außerdem am Rande der • 22:00 - 07:00 Uhr: 1,00 € Altstadt von Treysa eine Tagespreis: Begegnungsstätte (Steingasse 48, 24 Stunden: 22,00 € 34613 Schwalmstadt-Treysa) und ist Mitglied im Diakonischen Werk Hessen. -

Diegelmann Märchenhaft Radeln Märchenhaft Radeln
Kartentitel_A_LayoutKartentitel_A_Layout 1 1 08.02.2016 08.02.2016 14:03 14:03 Seite Seite 1 1 Gastronomie am Bahnradweg Servicebetriebe für Radreparaturen Schwalmstadt-Treysa Sehenswürdigkeiten Schwälmer Brotladen/Cafe, Bahnhofstraße 38, Tel.: (0 66 91) 91 88 07 Gasthaus „Am Brauhaus“, Ascherröder Str. 1, Tel.: (0 66 91) 15 59 Schwalmstadt-Treysa Schwalmstadt-Ziegenhain Fischi`s Bike Box, Stephanstr. 10, Tel.: (0 66 91) 91 61 88 Hotel/ Restaurant Rosengarten, Muhlystr. 3, Tel.: (0 66 91) 94 70-0 Alex‘s Fahrrad-Shop, Marienburger Straße 30, Tel.: (06691) 22 5 29 Hotel/ Restaurant Landgraf, Landgraf-Philipp-Str. 3, Tel.: (0 66 91) 40 83 Restaurant „Zum Schwälmer“, Wiederholdstr. 4, Tel.: (0 66 91) 38 81 Totenkirche, Treysa Schwälmer Brotladen/Cafe, Landgraf-Philipp-Straße 9, Tel.: (06691) 9215542 Marktplatz, Johannisbrunnen, Märchenfiguren, Treysa Schwalmstadt La Copa, Weinstube und Cafe, Paradeplatz 2, Tel.: (0 66 91) 9 27 01 64 Museum der Schwalm, Ziegenhain Kulturbahnhof Schwalm, Erich-Rohde-Str. 1, Tel.: (0 66 91) 806 10-0 Schwalmstadt Wasserfestung Ziegenhain Hotel/Restaurant Hof Weidelbach, Am Weidelbach, Tel. (0 66 91) 4726 Tourist-Info, Ziegenhain Museum und Gedenkstätte, Trutzhain Cafe im originellen Scheunenambiente Willingshausen-Zella mit großerFam. Auswahl Schimek an hausgemachtem • Stadtpark 1Kuchen (direkt am Radweg) Willi. Landgasthof Bechtel, Vor der Brücke 4, Tel.: (0 66 91) 94 65-0 1a Fahrradservice Diegelmann Niederrheinische Str. 27, Tel.: (0 66 94) 78 78 Biergarten „Unter34626 der Neukirchen Linde“ zum • Tel.Genießen 06694 der Sommerabende 247 Neukirchen bei einem guten Glas Wein oder einem frischen Pils Landhotel Combecher/ Biergarten, Kurhessenstr. 32, Tel.: (0 66 94) 9 78-0 Öffnungszeiten: Di.