GAYANA Assessing Climatic and Intrinsic Factors That Drive Arthropod
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Zootaxa, Pseudasthenes, a New Genus of Ovenbird (Aves
Zootaxa 2416: 61–68 (2010) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2010 · Magnolia Press ISSN 1175-5334 (online edition) Pseudasthenes, a new genus of ovenbird (Aves: Passeriformes: Furnariidae) ELIZABETH DERRYBERRY,1 SANTIAGO CLARAMUNT,1 KELLY E. O’QUIN,1,2 ALEXANDRE ALEIXO,3 R. TERRY CHESSER,4 J. V. REMSEN JR.1 & ROBB T. BRUMFIELD1 1Museum of Natural Science and Department of Biological Sciences, Louisiana State University, Baton Rouge, LA 70803 2Behavior Ecology Evolution Systematics Program, University of Maryland, College Park, MD 20742 3Coordenação de Zoologia, Museu Paraense Emílio Goeldi, Caixa Postal 399, CEP 66040-170, Belém, Pará, Brazil 4USGS Patuxent Wildlife Research Center, National Museum of Natural History, Smithsonian Institution, P.O. Box 37012, Washington, DC 20013 Abstract Phylogenetic analysis of the family Furnariidae (Aves: Passeriformes) indicates that the genus Asthenes is polyphyletic, consisting of two groups that are not sister taxa. Pseudasthenes, a new genus of ovenbird, is described for one of these groups. The four species included in the new genus, formerly placed in Asthenes, are P. humicola, P. patagonica, P. steinbachi, and P. cactorum. Key words: Asthenes, Oreophylax, Schizoeaca, phylogeny, taxonomy Asthenes Reichenbach 1853, a genus of the Neotropical avian family Furnariidae, currently contains 22 species of small ovenbirds restricted to Andean and southern South American temperate and subtropical regions, where they inhabit open areas dominated by rocks, shrubs and grasses (Remsen 2003). Members of the genus, commonly known as canasteros, are extremely diverse in behavior, ecology, and nest architecture, suggesting that Asthenes is not monophyletic (Pacheco et al. 1996; Zyskowski & Prum 1999; Remsen 2003; Vasconcelos et al. -

For the Love of Insects
For the Love of Insects “In terms of biomass and their interactions with other terrestrial organisms, insects are the most important group of terrestrial animals.” --Grimaldi and Engel, 2005 Outline • The Most Successful Animals on Earth: a Brief (Entomological) Journey through Time • Insect Physiology and Development • Common Insects and their Identification Whence and Whither: Insect Origins and Evolution Before diversity, there was evolution… A ~500 million year journey… Silurian • Insect Flight: 400 mya • Modern insect orders: 250 mya • Primitive mammals: 120 mya • Modern mammals: 60 mya The Jointed Animals Phylum: Arthropoda • 75% of all species on earth are arthropods • Internal/External specialization of body parts = tagmosis • Hardened exoskeleton • Articulated body plates • Paired, jointed appendages sciencenewsjournal.com Tagmosis: highly specialized body segments found in all arthropods; insects: head, thorax, abdomen; spiders: cephalothorax and opisthosoma Epiclass HEXAPODA: Late Silurian/Early Devonian Class Entognatha Order Diplura Ellipura Order Protura Order Collembola Class Insecta (= Ectognatha) Hexapoda • 6 legs; 11 abdominal segments (or fewer) taxondiversity.fieldofscience.com • Entognatha: Protura, Diplura, and Collembola • Ectognatha: Insects The First Insects: Apterygota Archaeognatha: The Jumping Bristletails • ~500 spp. worldwide; wide range of habitats; • 4 Families (2 extinct) which occur mostly in rocky habitats • Mostly detritovores, but scavenge dead arthropods or eat exuviae; • Indirect mating behavior; -

White-Throated Cacholote Pseudoseisura Gutturalis And
Cotinga 35 Observations on breeding of two Furnariidae in Patagonia: White-throated Cacholote Pseudoseisura gutturalis and Patagonian Canastero Pseudasthenes patagonica Kaspar Delhey and Cristian Hernán Fulvio Pérez Received 30 October 2011; final revision accepted 28 June 2012 Cotinga 35 (2013): 45–49 Describimos aspectos básicos de la biología reproductiva de dos especies de Furnariidae endémicas de la Argentina: el Cacholote Pardo Pseudoseisura gutturalis y el Canastero Patagónico Pseudasthenes patagonica. Además, documentamos el desarrollo de los pichones mediante fotografías y mediciones de peso. Hicimos el seguimiento de un nido de Cacholote Pardo y tres nidos de Canastero Patagónico, todos ubicados sobre arbustos espinosos a baja altura, en cercanía de Puerto Madryn, Chubut, Argentina. Ambas especies nidifican en nidos cerrados construidos con palitos espinosos. La puesta del Cacholote Pardo fue de cuatro huevos que fueron incubados durante 18 días. Los pichones permanecieron en el nido por 21 días antes de abandonar el nido de manera exitosa. Un nido del Canastero Patagónico fue seguido desde el inicio de su construcción, que llevó un mínimo de 20 días. La puesta fue de tres (dos nidos) o cuatro huevos de color blanco depositados en la cámara de incubación forrada con material vegetal blando. Los huevos eclosionaron luego de 15–16 días de incubación. Un nido fue depredado durante incubación, otro 4–5 días luego de la eclosión de los huevos y el tercero probablemente haya sido exitoso, abandonando los pichones el nido alrededor de 15 días luego de la eclosión. La duración de los períodos de incubación y permanencia en el nido de los pichones son similares a los de otras especies de los géneros Pseudoseisura y Asthenes. -

Evolution of the Insects David Grimaldi and Michael S
Cambridge University Press 0521821495 - Evolution of the Insects David Grimaldi and Michael S. Engel Frontmatter More information EVOLUTION OF THE INSECTS Insects are the most diverse group of organisms to appear in the 3-billion-year history of life on Earth, and the most ecologically dominant animals on land. This book chronicles, for the first time, the complete evolutionary history of insects: their living diversity, relationships, and 400 million years of fossils. Whereas other volumes have focused on either living species or fossils, this is the first comprehensive synthesis of all aspects of insect evolution. Current estimates of phylogeny are used to interpret the 400-million-year fossil record of insects, their extinctions, and radiations. Introductory sections include the living species, diversity of insects, methods of reconstructing evolutionary relationships, basic insect structure, and the diverse modes of insect fossilization and major fossil deposits. Major sections cover the relationships and evolution of each order of hexapod. The book also chronicles major episodes in the evolutionary history of insects: their modest beginnings in the Devonian, the origin of wings hundreds of millions of years before pterosaurs and birds, the impact that mass extinctions and the explosive radiation of angiosperms had on insects, and how insects evolved the most complex societies in nature. Evolution of the Insects is beautifully illustrated with more than 900 photo- and electron micrographs, drawings, diagrams, and field photographs, many in full color and virtually all original. The book will appeal to anyone engaged with insect diversity: professional ento- mologists and students, insect and fossil collectors, and naturalists. David Grimaldi has traveled in 40 countries on 6 continents collecting and studying recent species of insects and conducting fossil excavations. -

First Survey of Collembola (Hexapoda: Entognatha) Fauna in Soil of Archipelago Fernando De Noronha, Brazil Estevam C
First survey of Collembola (Hexapoda: Entognatha) fauna in soil of Archipelago Fernando de Noronha, Brazil Estevam C. Araujo De Lima1, * and Douglas Zeppelini1,2, Collembola (Hexapoda: Entognatha) is one of the most abundant Table 1. Collembola recorded on the Fernando de Noronha archipelago, Brazil. and widely distributed taxa among terrestrial Hexapoda (Hopkin 1997). Collection localites were: a sandy beach (SB), soil on the slope of a cliff (SC) and the Soil in the forest at the hilltop (SF). World distribution was summarized for Collembola specimens are found in almost all habitats, excluding each species as follows: Boreal (Bor) include regions 1–8, Neotropical (Neo) re- aquatic environments below the surface firm where their occurrence is gions 24–30, South African (Saf) region 31, Paleotropical (Pal) regions 9–23, Aus- rare or accidental. The greatest diversity and abundance of these spe- tralian (Aus) regions 32–34, and Antarctic (Ant) regions 35–37. Species distributed cies occurs in soil and in adjacent microhabitats, especially where there in at least, in 4 of the major regions (Neo, Pal, etc.) are considered to be cosmo- is much organic matter (Zeppelini et al. 2008). The potential value of politan (Cos). Species distribution restricted to Northeast and Central Brazil (NCB), restricted to Fernando de Noronha (RFN) and doubtful distribution Record (?). Collembola as biological indicators of soil health and ecosystem quality is increasingly recognized and therefore knowledge of the diversity of Localities Collembola becomes useful in the development of conservation strate- World gies and environmental monitoring (Stork & Eggleton 1992; Zeppelini Taxa SB SC SF distribution et al. -

Collembola (Entognatha) from East Africa
Eur. J. Entomol. 95: 217-237, 1998 ISSN 1210-5759 Collembola (Entognatha) from East Africa W anda M. WEINER1 and Judith NAJT2 1 Institute of Systematics and Evolution of Animals, Polish Academy of Sciences, Slawkowska 17, PL-31016 Krakdw, Poland 2EP 90 du CNRS, Laboratoire d’Entomologie, Muséum National d’Histoire Naturelle, 45, rue Buffon, F-75005 Paris, France Collembola, Hypogastruridae, Odontellidae, Neanuridae, Onychiuridae, Isotomidae, East Africa, identification keys, new species, redescription Abstract. Materials of Hypogastruridae, Odontellidae, Neanuridae, Onychiuridae and Isotomidae from East Africa were studied. Several new species are described: Acherontiella kowalskiorum sp. n., Furcu- lanurida grandcolasorum sp. n., Stachorutes dallaii sp. n., and Paleonura cassagnaui sp. n. Friesea vtorovi Tshelnokov, 1977 and Tullbergia kilimanjarica (Delamare Deboutteville, 1953) are redescribed. Stachorutes arlei (Thibaud & Massoud, 1980) is a new combination. Identification keys for Friesea Dalla Torre, 1895 with 2 + 2 eyes and Stachorutes Dallai, 1973 are given. INTRODUCTION The last systematic account on the Collembola of East Africa was presented by Dehar- veng and Diaz in 1984 with a review of all references concerning this region. The present study is based on the material collected in Tanzania and Kenya. Abbreviations . ISEA - Institute of Systematics and Evolution of Animals, Polish Academy of Sci ences, Krakdw, Poland; MNHN - Laboratoire d ’Entomologie, Muséum national d’Histoire naturelle, Paris, France. SYSTEMATIC ACCOUNT Family Hypogastruridae Ceratophysella denticulata Bagnall, 1941 M aterial examined . Tanzania, Ngorongoro Conservation Area, 2,200 m a.s.l., brink of the crater, dry forest near Sopa Lodge, 28.viii.1996, lgt. B. & K. Kowalski, 2 specimens: $ and 6 juv. Geographical distribution . -

Descargar Archivo
ISSN (impreso) 0327-0017 - ISSN (on-line) 1853-9564 NNótulótulasas FAUNÍSTICAS 290 Segunda Serie Junio 2020 AVES DE LA PREPUNA DEL NOROESTE DE ARGENTINA Patricia Capllonch1,2, Fernando Diego Ortiz1,3 y Rebeca Lobo Allende1, 4 1Centro Nacional de Anillado de Aves (CENAA), Facultad de Ciencias Naturales e Instituto Miguel Lillo, Universidad Nacional de Tucumán, Miguel Lillo 205, San Miguel de Tucumán (4000) Tucumán, Argentina. [email protected] 2Cátedra de Biornitología Argentina, Facultad de Ciencias Naturales e Instituto Miguel Lillo, Universidad Nacional de Tucumán, Miguel Lillo205, San Miguel de Tucumán (4000), Tucumán, Argentina. 3Centro de Rehabilitación de Aves Rapaces (CeRAR), Reserva Experimental Horco Molle, Facultad de Ciencias Naturales e Instituto Miguel Lillo, Universidad Nacional de Tucumán, Miguel Lillo 205, San Miguel de Tucumán (4000), Tucumán, Argentina. 4Universidad Nacional de Chilecito, Campus Los Sarmientos, Ruta Los Peregrinos s/n, Los Sarmientos, Chilecito, La Rioja. RESUMEN. Se analiza la avifauna en 11 localidades de la Prepuna, en el noroeste de la Argentina. Se registraron 165 especies de aves repartidas en 17 órdenes, 35 familias y 119 géneros. Esta riqueza representa el 17% de la avifauna del país, el 35% de la de Tucumán, el 29% de la de Jujuy y el 25% de la de Salta. Las localidades de Prepuna con mayor riqueza de aves fueron Cachi en la provincia de Salta, con 74 especies, El Molle, departamento Tafí, con 63 especies y El Arbolar, Colalao del Valle, con 60 especies, ambas en la provincia de Tucumán. Las especies con presencia constante o regular a lo largo del año con registros de captura en invierno y verano fueron 37 y solo 25 de ellas por su abundancia y constancia estacional son diagnósticas de la Prepuna argentina. -
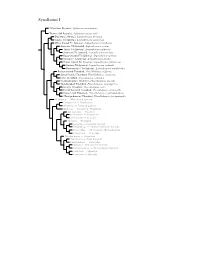
Synallaxini Species Tree
Synallaxini I ?Masafuera Rayadito, Aphrastura masafuerae Thorn-tailed Rayadito, Aphrastura spinicauda Des Murs’s Wiretail, Leptasthenura desmurii Tawny Tit-Spinetail, Leptasthenura yanacensis White-browed Tit-Spinetail, Leptasthenura xenothorax Araucaria Tit-Spinetail, Leptasthenura setaria Tufted Tit-Spinetail, Leptasthenura platensis Striolated Tit-Spinetail, Leptasthenura striolata Rusty-crowned Tit-Spinetail, Leptasthenura pileata Streaked Tit-Spinetail, Leptasthenura striata Brown-capped Tit-Spinetail, Leptasthenura fuliginiceps Andean Tit-Spinetail, Leptasthenura andicola Plain-mantled Tit-Spinetail, Leptasthenura aegithaloides Rufous-fronted Thornbird, Phacellodomus rufifrons Streak-fronted Thornbird, Phacellodomus striaticeps Little Thornbird, Phacellodomus sibilatrix Chestnut-backed Thornbird, Phacellodomus dorsalis Spot-breasted Thornbird, Phacellodomus maculipectus Greater Thornbird, Phacellodomus ruber Freckle-breasted Thornbird, Phacellodomus striaticollis Orange-eyed Thornbird, Phacellodomus erythrophthalmus ?Orange-breasted Thornbird, Phacellodomus ferrugineigula Hellmayrea — White-tailed Spinetail Coryphistera — Brushrunner Anumbius — Firewood-gatherer Asthenes — Canasteros, Thistletails Acrobatornis — Graveteiro Metopothrix — Plushcrown Xenerpestes — Graytails Siptornis — Prickletail Roraimia — Roraiman Barbtail Thripophaga — Speckled Spinetail, Softtails Limnoctites — ST Spinetail, SB Reedhaunter Cranioleuca — Spinetails Pseudasthenes — Canasteros Spartonoica — Wren-Spinetail Pseudoseisura — Cacholotes Mazaria – White-bellied -

2017 ESA, ESA Certification Corporation, and Entomological Foundation Awards
2017 ESA, ESA Certification Corporation, and Entomological Foundation Awards Each year the Entomological Society of America, the ESA Certification Corporation, and the Entomological Foundation provide annual honors and awards to recognize scientists, educators, early professionals, and students who have distinguished themselves through their contributions to entomology. For more information on the ESA, Certification Corporation, and Entomological Foundation Awards, visit www.entsoc.org/awards. ENTOMOLOGY 2017 | NOVEMBER 5–8 | DENVER, COLORADO 43 Awards: Sunday, November 5 2017 ESA, CERTIFICATION and ENTOMOLOGICAL FOUNDATION AWARDS Opening Plenary: ESA Fellows, ESA Honorary Members & Entomological Foundation Medal of Honor ESA’s 2017 Honorary Members and Fellows will be presented during the Opening Plenary Session on Sunday, November 5, 7:30 – 9:30 PM, and the Awards Breakfast on Tuesday, November 7, 7:30 – 9:00 AM. Both sessions will take place in the Four Seasons Ballroom of the Convention Center. Honorary Members Dr. Roger Moon became professor emeritus of entomology at the University of Honorary membership acknowledges those who have Minnesota in St. Paul after 36 years of served ESA for at least 20 years through significant service in research, teaching, and outreach. involvement in the affairs of the Society that has reached an He earned a Ph.D. in entomology from the extraordinary level. Candidates for this honor are selected University of California, Davis, in 1979. His research by the ESA Governing Board and then voted on by the ESA concerns the biology, ecology, and management of filth membership. flies, mosquitoes, bots, keds, lice, bugs, and mites that occur around livestock, wildlife, and people. -

The Genome of the Blind Soil-Dwelling and Ancestrally Wingless Dipluran Campodea Augens, a Key Reference Hexapod for Studying the Emergence of Insect Innovations
bioRxiv preprint doi: https://doi.org/10.1101/585695; this version posted June 29, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. The genome of the blind soil-dwelling and ancestrally wingless dipluran Campodea augens, a key reference hexapod for studying the emergence of insect innovations Mosè Manni1*, Felipe A. Simao1, Hugh M. Robertson2, Marco A. Gabaglio1, Robert M. Waterhouse3, Bernhard Misof4, Oliver Niehuis5, Nikolaus U. Szucsich6, Evgeny M. Zdobnov1* 1Department of Genetic Medicine and Development, University of Geneva Medical School, and Swiss Institute of Bioinformatics, Geneva, Switzerland. 2Department of Entomology, University of Illinois at Urbana-Champaign, Urbana, IL, USA. 3Department of Ecology and Evolution, University of Lausanne, and Swiss Institute of Bioinformatics, Lausanne, Switzerland. 4Center for Molecular Biodiversity Research, Zoological Research Museum Alexander Koenig, Bonn, Germany. 5Department of Evolutionary Biology and Ecology, Albert Ludwig University, Institute of Biology I (Zoology), Freiburg, Germany. 6Natural History Museum Vienna, 3rd Zoological Dept., Vienna, Austria. *Authors for Correspondence: Evgeny M. Zdobnov, email: [email protected] Mosè Manni, email: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/585695; this version posted June 29, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. -

Collembola (Springtails) (Arthropoda: Hexapoda: Entognatha) Found in Scrapings from Individuals Diagnosed with Delusory Parasitosis
J. New York Entomol. Soc. 112(1):87–95, 2004 COLLEMBOLA (SPRINGTAILS) (ARTHROPODA: HEXAPODA: ENTOGNATHA) FOUND IN SCRAPINGS FROM INDIVIDUALS DIAGNOSED WITH DELUSORY PARASITOSIS 1 2 DEBORAH Z. ALTSCHULER, MICHAEL CRUTCHER, MD, MPH, FACPM, 3 1 NECULAI DULCEANU, DVM, PHD(DECEASED), BETH A. CERVANTES, 4 5 CRISTINA TERINTE, MD, PHD AND LOUIS N. SORKIN, BCE 1National Pediculosis Association, 50 Kearney Road, Needham, Massachusetts 02494; 2Commissioner of Health, Oklahoma State Department of Health, 1000 NE 10th Street, Oklahoma City, Oklahoma 73117; 3Department of Parasitology, University of Veterinary Medicine, Iasi, Romania; 4Department of Pathology, University of Medicine and Pharmacy, Iasi, Romania; and 5Division of Invertebrate Zoology, American Museum of Natural History, Central Park West at 79th Street, New York, New York 10024-5192 Abstract.—Twenty individuals diagnosed with delusory parasitosis participated in a single site clinical study under the auspices of the National Pediculosis Association (NPA) and the Oklahoma State Department of Health. The objective of this study was to determine if there were any common factors in skin scrapings collected from this population. These individuals, whose symptoms were originally attributed to lice or scabies, were part of a larger group reporting symptoms of stinging/biting and/or crawling to the NPA. Multiple skin scrapings from each person were microscopically examined. Any and all fields of view that appeared incongruous to normal human skin were digitally photographed. When the photographic images were initially evaluated, no common factor was identified. However, more extensive scrutiny using imaging software revealed evidence of Collembola in 18 of the 20 participants. Key words: Collembola, springtail, stinging, biting, crawling, Arthropoda, Hexapoda, Entognatha, pediculosis, human skin, lice and scabies. -

Ecological Drivers of Song Evolution in Birds: Disentangling the Effects of Habitat and Morphology
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Faculty Publications and Other Works -- Ecology and Evolutionary Biology Ecology and Evolutionary Biology 1-13-2018 Ecological Drivers of Song Evolution in Birds: Disentangling the Effects of Habitat and Morphology Elizabeth Perrault Derryberry University of Tennessee, Knoxville, [email protected] Nathalie Seddon University of Oxford, UK Graham Earnest Derryberry Louisiana State University Santiago Claramunt Louisiana State University Glenn Fairbanks Seeholzer Louisiana State University See next page for additional authors Follow this and additional works at: https://trace.tennessee.edu/utk_ecolpubs Recommended Citation Derryberry, Elizabeth Perrault, Nathalie Seddon, Graham Earnest Derryberry, Santiago Claramunt, Glenn Fairbanks Seeholzer, Robb Thomas Brumfield, and Joseph Andrew Tobias. “Ecological Drivers of Song Evolution in Birds: Disentangling the Effects of Habitat and Morphology.” Ecology and Evolution 8, no. 3 (2018). https://doi.org/10.1002/ece3.3760. This Article is brought to you for free and open access by the Ecology and Evolutionary Biology at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Faculty Publications and Other Works -- Ecology and Evolutionary Biology by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. Authors Elizabeth Perrault Derryberry, Nathalie Seddon, Graham Earnest Derryberry, Santiago Claramunt, Glenn Fairbanks