Immunogenicity 28 and 35 Has Multiple Effects on T Cell Type I IFN
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

IN TAX LEADERS WOMEN in TAX LEADERS | 4 AMERICAS Latin America
WOMEN IN TAX LEADERS THECOMPREHENSIVEGUIDE TO THE WORLD’S LEADING FEMALE TAX ADVISERS SIXTH EDITION IN ASSOCIATION WITH PUBLISHED BY WWW.INTERNATIONALTAXREVIEW.COM Contents 2 Introduction and methodology 8 Bouverie Street, London EC4Y 8AX, UK AMERICAS Tel: +44 20 7779 8308 4 Latin America: 30 Costa Rica Fax: +44 20 7779 8500 regional interview 30 Curaçao 8 United States: 30 Guatemala Editor, World Tax and World TP regional interview 30 Honduras Jonathan Moore 19 Argentina 31 Mexico Researchers 20 Brazil 31 Panama Lovy Mazodila 24 Canada 31 Peru Annabelle Thorpe 29 Chile 32 United States Jason Howard 30 Colombia 41 Venezuela Production editor ASIA-PACIFIC João Fernandes 43 Asia-Pacific: regional 58 Malaysia interview 59 New Zealand Business development team 52 Australia 60 Philippines Margaret Varela-Christie 53 Cambodia 61 Singapore Raquel Ipo 54 China 61 South Korea Managing director, LMG Research 55 Hong Kong SAR 62 Taiwan Tom St. Denis 56 India 62 Thailand 58 Indonesia 62 Vietnam © Euromoney Trading Limited, 2020. The copyright of all 58 Japan editorial matter appearing in this Review is reserved by the publisher. EUROPE, MIDDLE EAST & AFRICA 64 Africa: regional 101 Lithuania No matter contained herein may be reproduced, duplicated interview 101 Luxembourg or copied by any means without the prior consent of the 68 Central Europe: 102 Malta: Q&A holder of the copyright, requests for which should be regional interview 105 Malta addressed to the publisher. Although Euromoney Trading 72 Northern & 107 Netherlands Limited has made every effort to ensure the accuracy of this Southern Europe: 110 Norway publication, neither it nor any contributor can accept any regional interview 111 Poland legal responsibility whatsoever for consequences that may 86 Austria 112 Portugal arise from errors or omissions, or any opinions or advice 87 Belgium 115 Qatar given. -
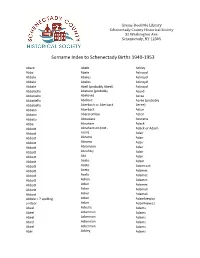
Surname Index to Schenectady Births 1940-1953
Grems-Doolittle Library Schenectady County Historical Society 32 Washington Ave. Schenectady, NY 12305 Surname Index to Schenectady Births 1940-1953 Abare Abele Ackley Abba Abele Ackroyd Abbale Abeles Ackroyd Abbale Abeles Ackroyd Abbale Abell (probably Abeel) Ackroyd Abbatiello Abelone (probably Acord Abbatiello Abelove) Acree Abbatiello Abelove Acree (probably Abbatiello Aberbach or Aberback Aeree) Abbato Aberback Acton Abbato Abercrombie Acton Abbato Aboudara Acucena Abbe Abraham Adack Abbott Abrahamson (not - Adack or Adach Abbott nson) Adair Abbott Abrams Adair Abbott Abrams Adair Abbott Abramson Adair Abbott Abrofsky Adair Abbott Abt Adair Abbott Aceto Adam Abbott Aceto Adamczak Abbott Aceto Adamec Abbott Aceto Adamec Abbott Acken Adamec Abbott Acker Adamec Abbott Acker Adamek Abbott Acker Adamek Abbzle = ? spelling Acker Adamkiewicz unclear Acker Adamkiewicz Abeel Ackerle Adams Abeel Ackerman Adams Abeel Ackerman Adams Abeel Ackerman Adams Abeel Ackerman Adams Abel Ackley Adams Grems-Doolittle Library Schenectady County Historical Society 32 Washington Ave. Schenectady, NY 12305 Surname Index to Schenectady Births 1940-1953 Adams Adamson Ahl Adams Adanti Ahles Adams Addis Ahman Adams Ademec or Adamec Ahnert Adams Adinolfi Ahren Adams Adinolfi Ahren Adams Adinolfi Ahrendtsen Adams Adinolfi Ahrendtsen Adams Adkins Ahrens Adams Adkins Ahrens Adams Adriance Ahrens Adams Adsit Aiken Adams Aeree Aiken Adams Aernecke Ailes = ? Adams Agans Ainsworth Adams Agans Aker (or Aeher = ?) Adams Aganz (Agans ?) Akers Adams Agare or Abare = ? Akerson Adams Agat Akin Adams Agat Akins Adams Agen Akins Adams Aggen Akland Adams Aggen Albanese Adams Aggen Alberding Adams Aggen Albert Adams Agnew Albert Adams Agnew Albert or Alberti Adams Agnew Alberti Adams Agostara Alberti Adams Agostara (not Agostra) Alberts Adamski Agree Albig Adamski Ahave ? = totally Albig Adamson unclear Albohm Adamson Ahern Albohm Adamson Ahl Albohm (not Albolm) Adamson Ahl Albrezzi Grems-Doolittle Library Schenectady County Historical Society 32 Washington Ave. -

Characterization of Visceral and Subcutaneous Adipose Tissue
J. Perinat. Med. 2016; 44(7): 813–835 Shali Mazaki-Tovi*, Adi L. Tarca, Edi Vaisbuch, Juan Pedro Kusanovic, Nandor Gabor Than, Tinnakorn Chaiworapongsa, Zhong Dong, Sonia S. Hassan and Roberto Romero* Characterization of visceral and subcutaneous adipose tissue transcriptome in pregnant women with and without spontaneous labor at term: implication of alternative splicing in the metabolic adaptations of adipose tissue to parturition DOI 10.1515/jpm-2015-0259 groups (unpaired analyses) and adipose tissue regions Received July 27, 2015. Accepted October 26, 2015. Previously (paired analyses). Selected genes were tested by quantita- published online March 19, 2016. tive reverse transcription-polymerase chain reaction. Abstract Results: Four hundred and eighty-two genes were differ- entially expressed between visceral and subcutaneous Objective: The aim of this study was to determine gene fat of pregnant women with spontaneous labor at term expression and splicing changes associated with par- (q-value < 0.1; fold change > 1.5). Biological processes turition and regions (visceral vs. subcutaneous) of the enriched in this comparison included tissue and vascu- adipose tissue of pregnant women. lature development as well as inflammatory and meta- Study design: The transcriptome of visceral and abdomi- bolic pathways. Differential splicing was found for 42 nal subcutaneous adipose tissue from pregnant women at genes [q-value < 0.1; differences in Finding Isoforms using term with (n = 15) and without (n = 25) spontaneous labor Robust Multichip Analysis scores > 2] between adipose was profiled with the Affymetrix GeneChip Human Exon tissue regions of women not in labor. Differential exon 1.0 ST array. Overall gene expression changes and the dif- usage associated with parturition was found for three ferential exon usage rate were compared between patient genes (LIMS1, HSPA5, and GSTK1) in subcutaneous tissues. -
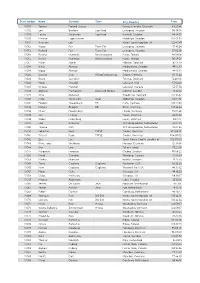
Start Number Name Surname Team City, Country Time 11001 Tommy
Start number Name Surname Team City, Country Time 11001 Tommy Frølund Jensen Kongens lyngby, Denmark 89:20:46 11002 Emil Boström Lag Nord Linköping, Sweden 98:19:04 11003 Lovisa Johansson Lag Nord Burträsk, Sweden 98:19:02 11005 Maurice Lagershausen Huddinge, Sweden 102:01:54 11011 Hyun jun Lee Korea, (south) republic of 122:40:49 11012 Viggo Fält Team Fält Linköping, Sweden 57:45:24 11013 Richard Fält Team Fält Linköping, Sweden 57:45:28 11014 Rasmus Nummela Rackmannarna Pjelax, Finland 98:59:06 11015 Rafael Nummela Rackmannarna Pjelax, Finland 98:59:06 11017 Marie Zølner Hillerød, Denmark 82:11:49 11018 Niklas Åkesson Mellbystrand, Sweden 99:45:22 11019 Viggo Åkesson Mellbystrand, Sweden 99:45:22 11020 Damian Sulik #StopComplaining Siegen, Germany 101:13:24 11023 Bruno Svendsen Tylstrup, Denmark 74:48:08 11024 Philip Oswald Lakeland, USA 55:52:02 11025 Virginia Marshall Lakefield, Canada 55:57:54 11026 Andreas Mathiasson Backyard Heroes Ödsmål, Sweden 75:12:02 11028 Stine Andersen Brædstrup, Denmark 104:35:37 11030 Patte Johansson Tiger Södertälje, Sweden 80:24:51 11031 Frederic Wiesenbach BB Dahn, Germany 101:21:50 11032 Romea Brugger BB Berlin, Germany 101:22:44 11034 Oliver Freudenberg Heyda, Germany 75:07:48 11035 Jan Hennig Farum, Denmark 82:11:50 11036 Robert Falkenberg Farum, Denmark 82:11:51 11037 Edo Boorsma Sint annaparochie, Netherlands 123:17:34 11038 Trijneke Stuit Sint annaparochie, Netherlands 123:17:35 11040 Sebastian Keck TATSE Gieäen, Germany 104:08:01 11041 Tatjana Kage TATSE Gieäen, Germany 104:08:02 11042 Eun Lee -

BELGIUM NAME № YEAR Abbeloos, Antoine & Flore (Devos) 944 1975 Abras, Joseph & Jeanne (Delage) 12468 2012 Aelbers, Mathieu H
Righteous Among the Nations Honored by Yad Vashem by 1 January 2020 BELGIUM NAME № YEAR Abbeloos, Antoine & Flore (Devos) 944 1975 Abras, Joseph & Jeanne (Delage) 12468 2012 Aelbers, Mathieu H. & Maria (Alberts) 2794 1984 Aendekerk, Petrus Johannes H. 3109 1985 Aerts Jean 10604 2005 Alardo, Victor & Ida; ch: Marcelle, Marie-Louise, 5483 1992 Joseph Alleman, Jean-Baptiste & Augusta 6978 1996 Amelot, Adele 7357 1996 Anciaux, Emile & Josephine 6466 1994 Andre Emile & daughter Madeleine 8864 2000 Andre Emilie & sister Juliette 8864.1 2000 Andre, Joseph 486 1968 Andre, Paul & Jeanne 2407 1983 Andries, Joseph Jean & Marie (Vanden Bergh) 13158 2015 Andriesse-Renard, Paule 7065 1996 Aneuse, Nelly (Mother Julienne Aneuse) 13034.3 2015 Annaert, Petrus & Isabella, her mother Catherine 11130.1 2007 Steenput-Jacobs Ansiaux, Nicolas J. & Marie Celine 4031.1 1988 Antonis-Maluin, Rene & Leontine (Depraetere) 4435 1989 Arcq, Georgees & Lydie 8432 1999 Arekens, Marie 7465.1 1997 Arnauts, Marie T.E. (Mere Ursule) 11793 2010 Arnoldy, Marcel & Celestine (Schyns) 11350 2008 Arnould, Fernand & Nelly (Bayard) 756 1972 Artus, Mathilde 3127 1985 Audenardt, Pieter & Victorine & daughter Germaine 5986 1994 (Van 't Hoff) Avaux, Evence & Irene (Grimee) 8306 1998 Baeck, Bernard & Arlette (Peeters) 11697 2009 Baeten, Antoon & Maria (Jaspers) 1748 1980 Baggen, Helene 9938 2003 Baichez, Fernand & Juliette-Marie 1638.4 2002 Bal, Auguste & Odile 8998 2000 Bamps, Louis & Elisa 9981 2003 Bams, Joannes & Elvira (Borgugnons) 13402 2017 Bartholomeus, Urbain & Marie-Louise 8910 -

Exploration, Disruption, Diaspora: Movement of Nuevomexicanos to Utah, 1776-1850 Linda C
University of New Mexico UNM Digital Repository American Studies ETDs Electronic Theses and Dissertations Spring 5-11-2019 Exploration, Disruption, Diaspora: Movement of Nuevomexicanos to Utah, 1776-1850 Linda C. Eleshuk Roybal Follow this and additional works at: https://digitalrepository.unm.edu/amst_etds Part of the American Studies Commons Recommended Citation Eleshuk Roybal, Linda C.. "Exploration, Disruption, Diaspora: Movement of Nuevomexicanos to Utah, 1776-1850." (2019). https://digitalrepository.unm.edu/amst_etds/80 This Dissertation is brought to you for free and open access by the Electronic Theses and Dissertations at UNM Digital Repository. It has been accepted for inclusion in American Studies ETDs by an authorized administrator of UNM Digital Repository. For more information, please contact [email protected]. Linda Catherine Eleshuk Roybal Candidate American Studies Department This dissertation is approved and it is acceptable in quality and form for publication: Approved by the Dissertation Committee: A.Gabriel Meléndez, Chair Kathleen Holscher Michelle Hall Kells Enrique Lamadrid i Exploration, Disruption, Diaspora: Movement Of Nuevomexicanos to Utah, 1776 – 1950 By Linda Catherine Eleshuk Roybal B.S. Psychology, Weber State College, 1973 B.S. Communications, Weber State University, 1982 M.S. English, Utah State University, 1997 DISSERTATION Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy In American Studies The University of New Mexico Albuquerque, New Mexico May 2019 ii Dedication This dissertation is dedicated to my children and grandchildren, born and yet to be — Joys of my life and ambassadors to a future I will not see, Con mucho cariño. iii Acknowledgements I am grateful to so many people who have shared their expertise and resources to bring this project to its completion. -

Measuring and Modeling of Axon Membrane Properties in Motor Neuron Disorders and Normal Subjects
1 2 1 Measuring and modeling of axon membrane properties in motor neuron disorders and normal subjects Maria Kovalchuk 2 Cover design Picture of a marshalling yard south to Hamburg (Germany) from Google Planet Earth, Inc., adopted by Natalia Solovyeva, Maria Kovalchuk Layout Natalia Solovyeva Printed by Buki Vedi ISBN 978-5-4465-2558-4 Copyright © 2019 M. Kovalchuk All rights reserved. No part of this publication may be reproduced, distributed or transmitted in any form or by any means, electronic or mechanical, including photocopy, recording, or any information storage or retrieval system, without permission in writing form from the author. The copyright of the articles that have been accepted for publication or that have already been published, has been trans- ferred to the respective journals. 3 Measuring and modeling of axon membrane properties in motor neuron disorders and normal subjects Meten en modelleren van axonmembraaneigenschappen bij motor neuron aandoeningen en gezonden (met een samenvatting in het Nederlands) Proefschrift ter verkrijging van de graad van doctor aan de Universiteit Utrecht op gezag van de rector magnificus, prof.dr. H.R.B.M Kummeling, ingevolge het besluit van het college voor promoties in het openbaar te verdedigen op donderdag 19 december 2019 des avonds te 6.00 uur door Maria Kovalchuk geboren op 5 oktober 1984 te Moskou, Rusland 4Promotor: Prof. dr. L.H. van den Berg Copromotoren: Dr. H. Franssen Dr. ir. B.T.H.M. Sleutjes Funding of the studies described in this thesis was provided by the European Federation of Neurological Societies and Prinses Beatrix Spierfonds. 5 The last process of reason is to recognize that there is an infinity of things which transcend it. -

Van Cott Information Services, Inc. Presents Woodwind Books, Music
Member: Van Cott Information Services, Inc. International Clarinet presents Association Woodwind Books, Music, CDs and More! This is our complete woodwind price list. Ask for the catalog for your area of interest or visit our web site at http://www.vcisinc.com for a complete description of all our products and downloadable catalogs. We accept orders by phone, fax, and the internet. Price List dated 4/01/20 – Price and Availability Subject to Change B115. Concerto for Bassoon & Orch (Pn Reduc) (Mancusi) ................... 19.95 Bassoon B112. Concerto for Bassoon and Orchestra in B-flat (PR) (JC Bach) ....... 17.95 B103. 2 Duos Concertants Op. 65 for 2 Bassoons (Fuchs) ...................... 18.50 B113. Concerto for Bassoon and Orchestra in E-flat (PR) (JC Bach) ......... 6.95 B098. 6 Sonates Op. 40 Vol. 1 for 2 Bassoons (Bodin de Boismortier) ..... 13.95 B118. Concerto for Bassoon & Orch Op. 75 (PR) (Weber/Waterhouse) .. 23.95 B099. 6 Sonates Op. 40 Vol. 2 for 2 Bassoons (Bodin de Boismortier) ..... 13.95 B170. Concerto for Contrabassoon Piano Reduction (Dorff) .................. 16.95 B100. 6 Sonates Vol. 1 for 2 Bassoons (Braun) ........................................ 10.95 B148. Concerto in F major Op. 75 for Bassoon & Pn (Weber/Sharrow) .. 14.75 B101. 6 Sonates Vol. 2 for 2 Bassoons (Braun) ........................................ 13.95 B147. Concerto for F Major for Bassoon and Piano (Hummel) .............. 14.95 B166. 8 More Original Jazz Duos for Clarinet and Bassoon (Curtis) ....... 14.50 B035. Concerto for Bassoon and Wind Ens. (bs & piano ed.) (Ewazen) .. 24.95 B128. 10 (Ten) Etudes for Bassoon (Lowman) .......................................... 4.00 B132. -

Iii. Administración Local
BOCM BOLETÍN OFICIAL DE LA COMUNIDAD DE MADRID B.O.C.M. Núm. 224 SÁBADO 20 DE SEPTIEMBRE DE 2014 Pág. 59 III. ADMINISTRACIÓN LOCAL AYUNTAMIENTO DE 24 FUENLABRADA RÉGIMEN ECONÓMICO No habiendo resultado posible practicar la notificación al interesado o su representante, conforme a lo dispuesto en el artículo 112 de la Ley General Tributaria, mediante el pre- sente anuncio se cita a los contribuyentes a continuación indicados para que comparezcan en el lugar que se indica, a fin de que les sean notificadas las actuaciones llevadas a cabo en los procedimientos tributarios que les afectan y que a continuación se señalan: Actuación que se notifica Providencia de apremio: en el uso de las facultades que me confiere el artículo 5.3.c) del Real Decreto 1174/1987, de 18 de septiembre, en relación con el artículo 70 del Real Decreto 939/2005, de 29 de julio, por el que se aprueba el Reglamento General de Recau- dación, no habiendo satisfecho el obligado tributario las deudas por el concepto, ejercicio e importe expresado, habiendo sido notificadas en legal forma y expirado el plazo de ingre- so en período voluntario, se inicia el devengo de los intereses de demora. Asimismo, con- forme a lo dispuesto en el artículo 167 de la Ley 58/2003, de 17 de diciembre, General Tri- butaria, procedo a liquidar los recargo establecidos en el artículo 28 de dicha Ley, recargo de apremio reducido del 10 por 100, si se satisface la totalidad de la deuda y el propio re- cargo dentro de los plazos previstos en el artículo 62.5 de la Ley General Tributaria, y recar- go ordinario del 20 por 100 cuando se satisfaga la deuda y el recargo fuera de dicho plazo. -

BB Surname Index
SURNAME Volume No. Page(s) Aamoth 42 3 28 Abbey 45 1 19 Abbey 45 2 25 Abbey 53 1 34 Abbot 42 2 19 Abbott 37 4 4 Abbott 38 4 11, 12 Abbott 49 3 29 Abbott 50 3 25 Abernathy 41 1 8 Abernathy 47 4 19 Abernethy 42 3 21, 22 Abernethy 44 1 29 Abernethy 51 2 42 Abington 39 2 6 Abraham 45 4 30 Abraham 50 4 14 Abram 43 1 22 Abrams 41 1 3 Abrams 47 1 3 Aby 53 1 8 Accerman 52 4 2 Accolti 51 1 31 Acerman 52 4 2 Acetlie 37 1 22 Acherman 52 4 2 Achor 40 2 9 Acker 48 3 8 Ackerman 44 1 2 Ackerman 44 3 5 Ackerman 49 2 36 Ackerman 52 4 2 Ackermen 52 4 2 Ackermin 52 4 2 Ackkermann 52 4 2 Ackley 49 1 31 Ackroyd 47 1 22 Acloa 41 3 28 Acotty 52 2 30 Acraman 52 4 2 Acreman 52 4 2 Acremen 52 4 2 Adair 39 2 16, 17 Adair 43 2 24 Adair 46 2 20 SURNAME Volume No. Page(s) Adair 46 3 19, 20 Adair 46 4 24 Adair 49 1 28 Adam 51 4 8 Adams 37 2 22 Adams 37 3 14, 19 Adams 38 3 4 Adams 39 2 6, 24 Adams 40 1 4, 21 Adams 42 2 21, 23 Adams 42 4 4, 23 Adams 43 1 18-20 Adams 43 3 15 Adams 43 4 24, 25 Adams 44 1 14, 32 Adams 44 4 4, 18, 22 Adams 45 1 12 Adams 45 2 14 Adams 46 2 22 Adams 46 3 20 Adams 46 4 24 Adams 48 3 6, 17 Adams 48 4 4 Adams 49 2 35, 38 Adams 49 3 27, 28, 29 Adams 50 3 33 Adams 51 4 34 Adams 52 3 36 Adams 52 4 2 Adams 53 1 33, 34 Adams 53 3 16, 18, 23 Adamson 39 1 26 Adamson 39 4 24 Adamson 48 3 6 Addison 43 2 9 Adison 39 2 17 Adkins 44 3 26 Adkins 48 4 26 Adkins 51 4 3 Adolph 38 1 4 Adolph 43 1 21 Adolph 45 3 30 Adolph 47 3 3 Adolph 48 4 26 Adolph 49 1 28 SURNAME Volume No. -

2011 Public Sector Salary Disclosure
Disclosure for 2011 under the Public Sector Salary Disclosure Act, 1996 Hospitals and Boards of Public Health This category includes Ontario Hospitals and Boards of Public Health. Some Boards of Public Health can be found under a municipality in the “Municipalities and Services” category. Divulgation pour 2011 en vertu de la Loi de 1996 sur la divulgation des traitements dans le secteur public Hôpitaux et Conseils de santé Cette catégorie contient les hôpitaux de l’Ontario et les Conseils de santé. Il se peut que certains Conseils de santé soient inclus dans la divulgation d’une municipalité dans la catégorie «Municipalités et services». Taxable Surname/Nom de Given Name/ Salary Paid/ Benefits/ Employer/Employeur famille Prénom Position/Poste Traitement Avant. impos. Alexandra Hospital, Ingersoll GARDNER LISA ANNE Director, Patient Services/Chief Nursing Officer$104,352.15 $793.45 Alexandra Hospital, Ingersoll JOANNE ACKERT Registered Nurse $100,982.19 $628.30 Alexandra Marine & General Hospital BEDARD RICHARD Chief Information Officer / Director Clinical Support$107,074.62 $604.56 Alexandra Marine & General Hospital MCGILLIVRAY SUSAN Chief Financial Officer / Director Human Resources$103,691.62 $586.26 Alexandra Marine & General Hospital TAYLOR CHERYL Director of Patient Services / Chief Nursing Executive$122,401.62 $692.16 Alexandra Marine & General Hospital THIBERT WILLIAM President / Chief Executive Officer$121,939.55 $163.40 Almonte General Hospital BLACKBURN RUBY Registered Nurse $103,179.27 $0.00 Almonte General Hospital LEAFLOOR -

Omics, Epigenetics, and Genome Editing Techniques for Food and Nutritional Security
plants Review OMICs, Epigenetics, and Genome Editing Techniques for Food and Nutritional Security Yuri V. Gogolev 1,2,* , Sunny Ahmar 3 , Bala Ani Akpinar 4 , Hikmet Budak 4 , Alexey S. Kiryushkin 5, Vladimir Y. Gorshkov 1,2, Goetz Hensel 6,7 , Kirill N. Demchenko 5 , Igor Kovalchuk 8, Freddy Mora-Poblete 3 , Tugdem Muslu 9 , Ivan D. Tsers 2 , Narendra Singh Yadav 8 and Viktor Korzun 2,10,* 1 Federal Research Center Kazan Scientific Center of Russian Academy of Sciences, Kazan Institute of Biochemistry and Biophysics, 420111 Kazan, Russia; [email protected] 2 Federal Research Center Kazan Scientific Center of Russian Academy of Sciences, Laboratory of Plant Infectious Diseases, 420111 Kazan, Russia; [email protected] 3 Institute of Biological Sciences, University of Talca, 1 Poniente 1141, Talca 3460000, Chile; [email protected] (S.A.); [email protected] (F.M.-P.) 4 Montana BioAg Inc., Missoula, MT 59802, USA; [email protected] (B.A.A.); [email protected] (H.B.) 5 Laboratory of Cellular and Molecular Mechanisms of Plant Development, Komarov Botanical Institute of the Russian Academy of Sciences, 197376 Saint Petersburg, Russia; [email protected] (A.S.K.); [email protected] (K.N.D.) 6 Centre for Plant Genome Engineering, Institute of Plant Biochemistry, Heinrich-Heine-University, 40225 Dusseldorf, Germany; [email protected] 7 Centre of the Region Haná for Biotechnological and Agricultural Research, Czech Advanced Technology and Research Institute, Palacký University Olomouc, Citation: Gogolev, Y.V.; Ahmar, S.; 78371 Olomouc, Czech Republic 8 Akpinar, B.A.; Budak, H.; Kiryushkin, Department of Biological Sciences, University of Lethbridge, Lethbridge, AB T1K 3M4, Canada; [email protected] (I.K.); [email protected] (N.S.Y.) A.S.; Gorshkov, V.Y.; Hensel, G.; 9 Faculty of Engineering and Natural Sciences, Sabanci University, 34956 Istanbul, Turkey; Demchenko, K.N.; Kovalchuk, I.; [email protected] Mora-Poblete, F.; et al.