Base Units All Measurements Consist of Two Parts: A. the Number B. The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lesson 1: Length English Vs
Lesson 1: Length English vs. Metric Units Which is longer? A. 1 mile or 1 kilometer B. 1 yard or 1 meter C. 1 inch or 1 centimeter English vs. Metric Units Which is longer? A. 1 mile or 1 kilometer 1 mile B. 1 yard or 1 meter C. 1 inch or 1 centimeter 1.6 kilometers English vs. Metric Units Which is longer? A. 1 mile or 1 kilometer 1 mile B. 1 yard or 1 meter C. 1 inch or 1 centimeter 1.6 kilometers 1 yard = 0.9444 meters English vs. Metric Units Which is longer? A. 1 mile or 1 kilometer 1 mile B. 1 yard or 1 meter C. 1 inch or 1 centimeter 1.6 kilometers 1 inch = 2.54 centimeters 1 yard = 0.9444 meters Metric Units The basic unit of length in the metric system in the meter and is represented by a lowercase m. Standard: The distance traveled by light in absolute vacuum in 1∕299,792,458 of a second. Metric Units 1 Kilometer (km) = 1000 meters 1 Meter = 100 Centimeters (cm) 1 Meter = 1000 Millimeters (mm) Which is larger? A. 1 meter or 105 centimeters C. 12 centimeters or 102 millimeters B. 4 kilometers or 4400 meters D. 1200 millimeters or 1 meter Measuring Length How many millimeters are in 1 centimeter? 1 centimeter = 10 millimeters What is the length of the line in centimeters? _______cm What is the length of the line in millimeters? _______mm What is the length of the line to the nearest centimeter? ________cm HINT: Round to the nearest centimeter – no decimals. -

Units and Magnitudes (Lecture Notes)
physics 8.701 topic 2 Frank Wilczek Units and Magnitudes (lecture notes) This lecture has two parts. The first part is mainly a practical guide to the measurement units that dominate the particle physics literature, and culture. The second part is a quasi-philosophical discussion of deep issues around unit systems, including a comparison of atomic, particle ("strong") and Planck units. For a more extended, profound treatment of the second part issues, see arxiv.org/pdf/0708.4361v1.pdf . Because special relativity and quantum mechanics permeate modern particle physics, it is useful to employ units so that c = ħ = 1. In other words, we report velocities as multiples the speed of light c, and actions (or equivalently angular momenta) as multiples of the rationalized Planck's constant ħ, which is the original Planck constant h divided by 2π. 27 August 2013 physics 8.701 topic 2 Frank Wilczek In classical physics one usually keeps separate units for mass, length and time. I invite you to think about why! (I'll give you my take on it later.) To bring out the "dimensional" features of particle physics units without excess baggage, it is helpful to keep track of powers of mass M, length L, and time T without regard to magnitudes, in the form When these are both set equal to 1, the M, L, T system collapses to just one independent dimension. So we can - and usually do - consider everything as having the units of some power of mass. Thus for energy we have while for momentum 27 August 2013 physics 8.701 topic 2 Frank Wilczek and for length so that energy and momentum have the units of mass, while length has the units of inverse mass. -

Measuring in Metric Units BEFORE Now WHY? You Used Metric Units
Measuring in Metric Units BEFORE Now WHY? You used metric units. You’ll measure and estimate So you can estimate the mass using metric units. of a bike, as in Ex. 20. Themetric system is a decimal system of measurement. The metric Word Watch system has units for length, mass, and capacity. metric system, p. 80 Length Themeter (m) is the basic unit of length in the metric system. length: meter, millimeter, centimeter, kilometer, Three other metric units of length are themillimeter (mm) , p. 80 centimeter (cm) , andkilometer (km) . mass: gram, milligram, kilogram, p. 81 You can use the following benchmarks to estimate length. capacity: liter, milliliter, kiloliter, p. 82 1 millimeter 1 centimeter 1 meter thickness of width of a large height of the a dime paper clip back of a chair 1 kilometer combined length of 9 football fields EXAMPLE 1 Using Metric Units of Length Estimate the length of the bandage by imagining paper clips laid next to it. Then measure the bandage with a metric ruler to check your estimate. 1 Estimate using paper clips. About 5 large paper clips fit next to the bandage, so it is about 5 centimeters long. ch O at ut! W 2 Measure using a ruler. A typical metric ruler allows you to measure Each centimeter is divided only to the nearest tenth of into tenths, so the bandage cm 12345 a centimeter. is 4.8 centimeters long. 80 Chapter 2 Decimal Operations Mass Mass is the amount of matter that an object has. The gram (g) is the basic metric unit of mass. -

Time in the Digital Age of Architecture
Master’s Thesis TIME IN THE DIGITAL AGE OF ARCHITECTURE by Katerina Konstantopoulos Graduate Degree of Architectural Engineering National Technical University Of Athens, 2011. Submitted to the department of architectural engineering in partial fulfillment of the requirements for The Postgraduate Degree of "Architecture – Spatial Design" Option: “Architectural Design – Space – Culture” At the National Technical University Of Athens - School of Architecture OCTOBER 2014 Master’s Committee: Advisor: Dimitris Papalexopoulos Co-Advisor: Giorgos Parmenidis Time in the Digital Age of Architecture Table of Contents ABSTARCT........................................................................................................................................3 INTRODUCTION..............................................................................................................................3 CULTURAL CONTEXT...................................................................................................................8 CONCEPTS OF SPACE AND TIME IN PHYSICS.....................................................................11 PRE-RELATIVE PHYSICS: NEWTON AND EUCLIDEAN GEOMETRY ................12 RELATIVE PHYSICS: EINSTEIN AND NON-EUCLIDEAN GEOMETRY ..............15 PHILOSOPHY OF TIME IN THE 20TH CENTURY.................................................................20 BERGSON.......................................................................................................................20 DELEUZE........................................................................................................................23 -

Measuring Distances Using Parallax 1 Introduction
Measuring Distances Using Parallax Name: Date: 1 Introduction How do astronomers know how far away a star or galaxy is? Determining the distances to the objects they study is one of the the most difficult tasks facing astronomers. Since astronomers cannot simply take out a ruler and measure the distance to any object, they have to use other methods. Inside the solar system, astronomers can simply bounce a radar signal off of a planet, asteroid or comet to directly measure the distance to that object (since radar is an electromagnetic wave, it travels at the speed of light, so you know how fast the signal travels{you just have to count how long it takes to return and you can measure the object's distance). But, as you will find out in your lecture sessions, some stars are hun- dreds, thousands or even tens of thousands of \light years" away. A light year is how far light travels in a single year (about 9.5 trillion kilometers). To bounce a radar signal of a star that is 100 light years away would require you to wait 200 years to get a signal back (remember the signal has to go out, bounce off the target, and come back). Obviously, radar is not a feasible method for determining how far away stars are. In fact, there is one, and only one direct method to measure the distance to a star: \parallax". Parallax is the angle that something appears to move when the observer looking at that object changes their position. By observing the size of this angle and knowing how far the observer has moved, one can determine the distance to the object. -

Orders of Magnitude (Length) - Wikipedia
03/08/2018 Orders of magnitude (length) - Wikipedia Orders of magnitude (length) The following are examples of orders of magnitude for different lengths. Contents Overview Detailed list Subatomic Atomic to cellular Cellular to human scale Human to astronomical scale Astronomical less than 10 yoctometres 10 yoctometres 100 yoctometres 1 zeptometre 10 zeptometres 100 zeptometres 1 attometre 10 attometres 100 attometres 1 femtometre 10 femtometres 100 femtometres 1 picometre 10 picometres 100 picometres 1 nanometre 10 nanometres 100 nanometres 1 micrometre 10 micrometres 100 micrometres 1 millimetre 1 centimetre 1 decimetre Conversions Wavelengths Human-defined scales and structures Nature Astronomical 1 metre Conversions https://en.wikipedia.org/wiki/Orders_of_magnitude_(length) 1/44 03/08/2018 Orders of magnitude (length) - Wikipedia Human-defined scales and structures Sports Nature Astronomical 1 decametre Conversions Human-defined scales and structures Sports Nature Astronomical 1 hectometre Conversions Human-defined scales and structures Sports Nature Astronomical 1 kilometre Conversions Human-defined scales and structures Geographical Astronomical 10 kilometres Conversions Sports Human-defined scales and structures Geographical Astronomical 100 kilometres Conversions Human-defined scales and structures Geographical Astronomical 1 megametre Conversions Human-defined scales and structures Sports Geographical Astronomical 10 megametres Conversions Human-defined scales and structures Geographical Astronomical 100 megametres 1 gigametre -

TESINA MIAD Modular Systems in Architecture.Indd
MODULAR SYSTEMS IN ARCHITECTURE An overview of modularity through case studies ESCUELA TÉCNICA SUPERIOR DE ARQUITECTURA LA SALLE TRABAJO FINAL DE MÁSTER PROYECTO INTEGRADO DE ARQUITECTURA MODULAR SYSTEMS IN ARCHITECTURE An overview of modularity through case studies ALUMNO/A DIRECTOR/A Cecilia Lema Scarsi David García Martinez 34°28’01” S 56°15’52” O A los de allí... Figure 1.1 George Nelson. Experimental House - Vitra Design Museum. 1950 6 ABSTRACT. Inside the atmosphere of architectural The paradigm that supports this work is based design often appears a singular concept mainly on the conceptual approach of modular systems surrounded by contradictions, vaguely defi ned and in architecture, primarily understood as those ambiguously applied. This term is used frequently architectural designs that comply with the property to qualify not one but multiple conditions of an of modularity in three major aspects simultaneously: architectural object, causing major dissonances and modular space indeterminacy, prefabricated modular becoming inevitably a question mark. building system and modular growth and adaptability. After having witnessed multiple approaches to the topic, it became clear that the same adjective was Synthetically, modular systems are identifi ed with their used to express different features of the objects search for undetermined spaces which dimensions in question. The overlap between the uses and and form are based on a strict modular design that meanings of the same term pointed out that there was subdivides the whole into equal parts. These systems a widespread and inaccurate usage of it: modularity in require the minimum number of prefabricated building architecture is from now on the concept under study. -

The Ubiquity of Phi in Human Culture & the Natural World
John Carroll University Carroll Collected Masters Essays Master's Theses and Essays 2020 THE UBIQUITY OF PHI IN HUMAN CULTURE & THE NATURAL WORLD Jennifer Bressler Follow this and additional works at: https://collected.jcu.edu/mastersessays Part of the Mathematics Commons THE UBIQUITY OF PHI IN HUMAN CULTURE & THE NATURAL WORLD An Essay Submitted to the Office of Graduate Studies College of Arts & Sciences of John Carroll University In Partial Fulfillment of the Requirements For the Degree of Master of Arts By Jennifer L. Bressler 2020 Table of Contents I. Introduction…………………………………………………………………………. 2 II. The Early Greeks…………………………………………………………………… 4 III. Algebraic Properties of the Golden Ratio………………………………………….. 11 IV. The Golden Rectangle…………………………………………………….……….. 20 V. Architecture & Design……………………………………………………………… 22 VI. Art………………………………………………………………………………….. 30 VII. Music……………………………………………………………………………….. 38 VIII. The Natural World………………………………………………………………….. 43 IX. Human Anatomy…………………………………………………………………… 52 X. Geometry…………………………………………………………………………… 56 XI. Conclusion……………………………………………………………………………65 1 I. INTRODUCTION What do rabbit breeding, tornadoes, the Chambered Nautilus, a pentagram, the rhythm of a heartbeat, apple seeds, the shape of a credit card, a pinecone, the human ear, DaVinci’s Last Supper, the structure of DNA, a light switch cover, and the structure of galaxies all have in common? Each relates to an extraordinary ratio that is highly efficient in nature, profoundly attractive to the human eye, and some claim, even divinely inspired. This special ratio is referred to as the “Golden Ratio” and is also known as the divine proportion, golden section, and golden mean. The Golden Ratio has a constant numeric value called “phi” (pronounced “FEE,” or “FI”) which is thought to be the most beautiful and astounding of all numbers. -

Brief History and Use of the ENGLISH and METRIC SYSTEMS of MEASUREMENT with a CHART of the MODERNIZED METRIC SYSTEM
AUG 13 1971 -^4 UNITED STATES DEPARTMENT OF COMMERCE 161670 C. R. SMITH, Secretary NATIONAL BUREAU OF STANDARDS / a. v. astin, Director Special Publication 304A. Issued 1968. lUj h Brief History and Use of THE ENGLISH AND METRIC SYSTEMS OF MEASUREMENT with a CHART OF THE MODERNIZED METRIC SYSTEM "Weights and measures may be ranked among the necessaries of life to every individual of human society. They enter into the eco- nomical arrangements and daily concerns of every family. They are necessary to every occupation of human industry; to the distribution and security of every species of property; to every transaction of trade and commerce ; to the labors of the husbandman ; to the in- genuity of the artificer; to the studies of the philosopher ; to the researches of the antiquarian, to the navigation of the mariner, and the marches of the soldier; to all the exchanges of peace, and all the operations of war." —JOHN QUINCY ADAMS When the American Colonies sepa- sembly of France on May 8, 1790, of the length of a great circle of the rated from the mother country to to enact a decree, sanctioned by Louis earth. This idea found favor with the assume among the nations of the XVI, which called upon the French French philosophers at the time of the earth a separate and individual sta- Academy of Sciences in concert with French Revolution, men who were tion, they retained, among other the Royal Society of London to "de- generally opposed to any vestige of things, the weights and measures duce an invariable standard for all of monarchical authority and preferred that had been used when they were the measures and all weights." Hav- a standard based on a constant of colonies, namely, the weights and ing already an adequate system of nature. -
Linear Measures: the Metric System
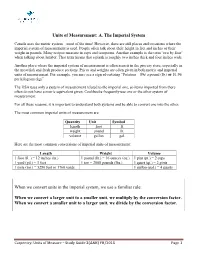
Units of Measurement: A. The Imperial System Canada uses the metric system – most of the time! However, there are still places and occasions where the imperial system of measurement is used. People often talk about their height in feet and inches or their weight in pounds. Many recipes measure in cups and teaspoons. Another example is the term ‘two by four’ when talking about lumber. That term means that a plank is roughly two inches thick and four inches wide. Another place where the imperial system of measurement is often seen is in the grocery store, especially in the meat/fish and fresh produce sections. Prices and weights are often given in both metric and imperial units of measurement. For example, you may see a sign advertising “Potatoes – 89¢ a pound (lb.) or $1.96 per kilogram (kg)”. The USA uses only a system of measurement related to the imperial one, so items imported from there often do not have a metric equivalent given. Cookbooks frequently use one or the other system of measurement. For all these reasons, it is important to understand both systems and be able to convert one into the other. The most common imperial units of measurement are: Quantity Unit Symbol length foot ft. weight pound lb. volume gallon gal. Here are the most common conversions of imperial units of measurement: Length Weight Volume 1 foot (ft. ) = 12 inches (in.) 1 pound (lb.) = 16 ounces (oz.) 1 pint (pt.) = 2 cups 1 yard (yd.) = 3 feet 1 ton = 2000 pounds (lbs.) 1 quart (qt.) = 2 pints 1 mile (mi.) = 5280 feet or 1760 yards 1 gallon (gal.) = 4 quarts When we convert units in the imperial system, we use a familiar rule: When we convert a larger unit to a smaller unit, we multiply by the conversion factor. -

Changing Metric Units
Measurement: 6-5 Changing Metric Units MAIN IDEA Change metric units of The lengths of two objects are shown below. length, capacity, and mass. Length Length Object (millimeters) (centimeters) New Vocabulary paper clip 45 4.5 metric system CD case 144 14.4 meter liter 1. Select three other objects. Find and record the width of all five gram kilogram objects to the nearest millimeter and tenth of a centimeter. 2. Compare the measurements of the objects, and write a rule that Math Online describes how to convert from millimeters to centimeters. glencoe.com 3. Measure the length of your classroom in meters. Make a • Extra Examples conjecture about how to convert this measure to centimeters. • Personal Tutor • Self-Check Quiz Explain. 1–3. See margin. The metric system is a decimal system of measures. The prefixes commonly used in this system are kilo-, centi-, and milli-. Meaning Meaning Prefix In Words In Numbers kilo- thousands 1,000 centi- hundredths 0.01 milli- thousandths 0.001 In the metric system, the base unit of length is the meter (m). Using the prefixes, the names of other units of length are formed. Notice that the prefixes tell you how the units relate to the meter. Unit Symbol Relationship to Meter kilometer km 1 km = 1,000 m 1 m = 0.001 km meter m 1 m = 1 m centimeter cm 1 cm = 0.01 m 1 m = 100 cm millimeter mm 1 mm = 0.001 m 1 m = 1,000 mm The liter (L) is the base unit of capacity, the amount of dry or liquid material an object can hold. -
Sg4-Units-Of-Measurement.Pdf
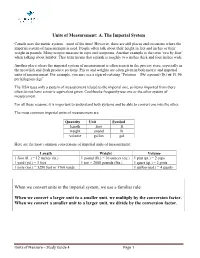
Units of Measurement: A. The Imperial System Canada uses the metric system – most of the time! However, there are still places and occasions where the imperial system of measurement is used. People often talk about their height in feet and inches or their weight in pounds. Many recipes measure in cups and teaspoons. Another example is the term ‘two by four’ when talking about lumber. That term means that a plank is roughly two inches thick and four inches wide. Another place where the imperial system of measurement is often seen is in the grocery store, especially in the meat/fish and fresh produce sections. Prices and weights are often given in both metric and imperial units of measurement. For example, you may see a sign advertising “Potatoes – 89¢ a pound (lb.) or $1.96 per kilogram (kg)”. The USA uses only a system of measurement related to the imperial one, so items imported from there often do not have a metric equivalent given. Cookbooks frequently use one or the other system of measurement. For all these reasons, it is important to understand both systems and be able to convert one into the other. The most common imperial units of measurement are: Quantity Unit Symbol length foot ft. weight pound lb. volume gallon gal. Here are the most common conversions of imperial units of measurement: Length Weight Volume 1 foot (ft. ) = 12 inches (in.) 1 pound (lb.) = 16 ounces (oz.) 1 pint (pt.) = 2 cups 1 yard (yd.) = 3 feet 1 ton = 2000 pounds (lbs.) 1 quart (qt.) = 2 pints 1 mile (mi.) = 5280 feet or 1760 yards 1 gallon (gal.) = 4 quarts When we convert units in the imperial system, we use a familiar rule: When we convert a larger unit to a smaller unit, we multiply by the conversion factor.