The Association of UNC13B Gene Polymorphisms and Diabetic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Role of Molecular Scaffolds at the Active Zone in Synaptic Vesicle Distribution and Release Probability
The Role of Molecular Scaffolds at the Active Zone in Synaptic Vesicle Distribution and Release Probability DISSERTATION To obtain the academic degree Doctor rerum naturalium (Dr. rer. nat.) submitted to the Department of Biology, Chemistry and Pharmacy Freie Universität Berlin Berlin, 2016 by Christina Beis (née Hollmann) This thesis was completed under the supervision of Prof. Dr. Stephan Sigrist from February 2011 to June 2016 at the Institute for Biology/ Genetics of Freie Universität Berlin, Germany. 1st reviewer: Prof. Dr. Stephan Sigrist 2nd reviewer: Prof. Dr. Hans-Joachim Pflüger Date of Defense: 21.11.2016 Statement of Authorship I hereby declare that the work presented in this thesis has been written independently and without inappropriate support. All sources of information are referenced. I hereby declare that this thesis has not been submitted, either in the same or in a different form, to this or any other university for a degree. ____________________________ Christina Beis Contents 1. Summary / Zusammenfassung .................................................................................................. 1 Summary....................................................................................................................................... 1 Zusammenfassung ........................................................................................................................ 3 2. Introduction .................................................................................................................................... -

Pharmacological Mechanisms Underlying the Hepatoprotective Effects of Ecliptae Herba on Hepatocellular Carcinoma
Hindawi Evidence-Based Complementary and Alternative Medicine Volume 2021, Article ID 5591402, 17 pages https://doi.org/10.1155/2021/5591402 Research Article Pharmacological Mechanisms Underlying the Hepatoprotective Effects of Ecliptae herba on Hepatocellular Carcinoma Botao Pan ,1 Wenxiu Pan ,2 Zheng Lu ,3 and Chenglai Xia 1,4 1Affiliated Foshan Maternity & Child Healthcare Hospital, Southern Medical University, Foshan 528000, China 2Department of Laboratory, Fifth People’s Hospital of Foshan, Foshan 528000, China 3Wuzhou Maternal and Child Health-Care Hospital, Wuzhou 543000, China 4School of Pharmaceutical Sciences, Southern Medical University, Guangzhou 510515, China Correspondence should be addressed to Zheng Lu; [email protected] and Chenglai Xia; [email protected] Received 30 January 2021; Revised 31 May 2021; Accepted 3 July 2021; Published 16 July 2021 Academic Editor: Hongcai Shang Copyright © 2021 Botao Pan et al. -is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Background. -e number of hepatocellular carcinoma (HCC) cases worldwide has increased significantly. As a traditional Chinese medicine (TCM) with a long history, Ecliptae herba (EH) has been widely used in HCC patients in China, but its hepatoprotective mechanism is still unclear. Methods. In this study, we applied a network pharmacology-based strategy and experimental ver- ification to systematically unravel the underlying mechanisms of EH against HCC. First, six active ingredients of EH were screened from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) by the ADME method. Subsequently, 52 potential targets of 6 active ingredients acting on HCC were screened from various databases, including TCMSP, DGIdb, SwissTargetPrediction, CTD, and GeneCards. -
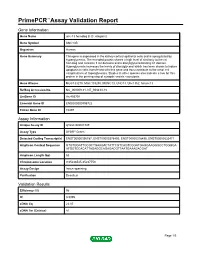
Primepcr™Assay Validation Report
PrimePCR™Assay Validation Report Gene Information Gene Name unc-13 homolog B (C. elegans) Gene Symbol UNC13B Organism Human Gene Summary This gene is expressed in the kidney cortical epithelial cells and is upregulated by hyperglycemia. The encoded protein shares a high level of similarity to the rat homolog and contains 3 C2 domains and a diacylglycerol-binding C1 domain. Hyperglycemia increases the levels of diacylglycerol which has been shown to induce apoptosis in cells transfected with this gene and thus contribute to the renal cell complications of hyperglycemia. Studies in other species also indicate a role for this protein in the priming step of synaptic vesicle exocytosis. Gene Aliases MGC133279, MGC133280, MUNC13, UNC13, Unc13h2, hmunc13 RefSeq Accession No. NC_000009.11, NT_008413.18 UniGene ID Hs.493791 Ensembl Gene ID ENSG00000198722 Entrez Gene ID 10497 Assay Information Unique Assay ID qHsaCID0007349 Assay Type SYBR® Green Detected Coding Transcript(s) ENST00000396787, ENST00000378495, ENST00000378496, ENST00000535471 Amplicon Context Sequence GTGTGGATTGCGCTGAAGACTATTCGTCAGTCGGATGAGGAAGGGCCTGGGGA ATGGTCCACATTAGAGGCAGAGACGTTAATGAAAGACGAT Amplicon Length (bp) 63 Chromosome Location 9:35236545-35237753 Assay Design Intron-spanning Purification Desalted Validation Results Efficiency (%) 96 R2 0.9995 cDNA Cq 22.07 cDNA Tm (Celsius) 81 Page 1/5 PrimePCR™Assay Validation Report gDNA Cq Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. Page 2/5 PrimePCR™Assay Validation Report -

Reduced Insulin Secretion Correlates with Decreased Expression of Exocytotic Genes in Pancreatic Islets from Patients with Type 2 Diabetes
Molecular and Cellular Endocrinology 364 (2012) 36–45 Contents lists available at SciVerse ScienceDirect Molecular and Cellular Endocrinology journal homepage: www.elsevier.com/locate/mce Reduced insulin secretion correlates with decreased expression of exocytotic genes in pancreatic islets from patients with type 2 diabetes Sofia A. Andersson a, Anders H. Olsson b, Jonathan L.S. Esguerra a, Emilia Heimann e, Claes Ladenvall c, Anna Edlund a, Albert Salehi d, Jalal Taneera c, Eva Degerman e, Leif Groop c, Charlotte Ling b, ⇑ Lena Eliasson a, a Islet Cell Exocytosis, Lund University Diabetes Centre, Department of Clinical Sciences Malmö, Lund University, Malmö, Sweden b Epigenetics and Diabetes, Lund University Diabetes Centre, Department of Clinical Sciences Malmö, Lund University, Malmö, Sweden c Diabetes and Endocrinology, Lund University Diabetes Centre, Department of Clinical Sciences Malmö, Lund University, Malmö, Sweden d Islet Cell Physiology, Lund University Diabetes Centre, Department of Clinical Sciences Malmö, Lund University, Malmö, Sweden e Department of Experimental Medical Sciences, Biomedical Center, Lund University, Lund, Sweden article info abstract Article history: Reduced insulin release has been linked to defect exocytosis in b-cells. However, whether expression of Received 14 December 2011 genes suggested to be involved in the exocytotic process (exocytotic genes) is altered in pancreatic islets Received in revised form 7 August 2012 from patients with type 2 diabetes (T2D), and correlate to insulin secretion, needs to be further investi- Accepted 13 August 2012 gated. Available online 23 August 2012 Analysing expression levels of 23 exocytotic genes using microarray revealed reduced expression of five genes in human T2D islets (v2 = 13.25; p < 0.001). -

Genomic and Transcriptome Analysis Revealing an Oncogenic Functional Module in Meningiomas
Neurosurg Focus 35 (6):E3, 2013 ©AANS, 2013 Genomic and transcriptome analysis revealing an oncogenic functional module in meningiomas XIAO CHANG, PH.D.,1 LINGLING SHI, PH.D.,2 FAN GAO, PH.D.,1 JONATHAN RUssIN, M.D.,3 LIYUN ZENG, PH.D.,1 SHUHAN HE, B.S.,3 THOMAS C. CHEN, M.D.,3 STEVEN L. GIANNOTTA, M.D.,3 DANIEL J. WEISENBERGER, PH.D.,4 GAbrIEL ZADA, M.D.,3 KAI WANG, PH.D.,1,5,6 AND WIllIAM J. MAck, M.D.1,3 1Zilkha Neurogenetic Institute, Keck School of Medicine, University of Southern California, Los Angeles, California; 2GHM Institute of CNS Regeneration, Jinan University, Guangzhou, China; 3Department of Neurosurgery, Keck School of Medicine, University of Southern California, Los Angeles, California; 4USC Epigenome Center, Keck School of Medicine, University of Southern California, Los Angeles, California; 5Department of Psychiatry, Keck School of Medicine, University of Southern California, Los Angeles, California; and 6Division of Bioinformatics, Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California Object. Meningiomas are among the most common primary adult brain tumors. Although typically benign, roughly 2%–5% display malignant pathological features. The key molecular pathways involved in malignant trans- formation remain to be determined. Methods. Illumina expression microarrays were used to assess gene expression levels, and Illumina single- nucleotide polymorphism arrays were used to identify copy number variants in benign, atypical, and malignant me- ningiomas (19 tumors, including 4 malignant ones). The authors also reanalyzed 2 expression data sets generated on Affymetrix microarrays (n = 68, including 6 malignant ones; n = 56, including 3 malignant ones). -

Whole-Genome Sequencing Reveals Genetic Variation in the Asian House Rat
INVESTIGATION Whole-Genome Sequencing Reveals Genetic Variation in the Asian House Rat Huajing Teng,*,†,‡ Yaohua Zhang,* Chengmin Shi,*,§ Fengbiao Mao,‡ Lingling Hou,‡ Hongling Guo,*,† Zhongsheng Sun,‡ and Jianxu Zhang*,1 *The State Key Laboratory of Integrated Management of Pest Insects and Rodents, Institute of Zoology, Chinese Academy of Sciences, 100101 Beijing, China, †University of Chinese Academy of Sciences, 100049 Beijing, China, § ‡ Beijing Institutes of Life Science, Chinese Academy of Sciences, 100101 Beijing, China, and Beijing Institute of Genomics, Chinese Academy of Sciences, 100101 Beijing, China ABSTRACT Whole-genome sequencing of wild-derived rat species can provide novel genomic resources, KEYWORDS which may help decipher the genetics underlying complex phenotypes. As a notorious pest, reservoir of human genetic pathogens, and colonizer, the Asian house rat, Rattus tanezumi, is successfully adapted to its habitat. However, landscape little is known regarding genetic variation in this species. In this study, we identified over 41,000,000 single- next-generation nucleotide polymorphisms, plus insertions and deletions, through whole-genome sequencing and bioinfor- sequencing matics analyses. Moreover, we identified over 12,000 structural variants, including 143 chromosomal inversions. Rattus tanezumi Further functional analyses revealed several fixed nonsense mutations associated with infection and immunity- single-nucleotide related adaptations, and a number of fixed missense mutations that may be related to anticoagulant resistance. polymorphisms A genome-wide scan for loci under selection identified various genes related to neural activity. Our whole- structural genome sequencing data provide a genomic resource for future genetic studies of the Asian house rat species variations and have the potential to facilitate understanding of the molecular adaptations of rats to their ecological niches. -

Starting a Molecular Systems View of Endocytosis
ANRV356-CB24-20 ARI 3 September 2008 19:11 ANNUAL Protein Kinases: Starting REVIEWS Further Click here for quick links to Annual Reviews content online, a Molecular Systems View including: • Other articles in this volume of Endocytosis • Top cited articles • Top downloaded articles • Our comprehensive search Prisca Liberali, Pauli Ram¨ o,¨ and Lucas Pelkmans Institute of Molecular Systems Biology, ETH Zurich, CH-8093 Zurich, Switzerland; email: [email protected] Annu. Rev. Cell Dev. Biol. 2008. 24:501–23 Key Words First published online as a Review in Advance on membrane trafficking, phosphorylation, signal transduction, July 3, 2008 complexity, nonlinear systems, genetical physics The Annual Review of Cell and Developmental Biology is online at cellbio.annualreviews.org Abstract This article’s doi: The field of endocytosis is in strong need of formal biophysical model- 10.1146/annurev.cellbio.041008.145637 ing and mathematical analysis. At the same time, endocytosis must be Copyright c 2008 by Annual Reviews. much better integrated into cellular physiology to understand the for- by Universitat Zurich- Hauptbibliothek Irchel on 04/05/13. For personal use only. All rights reserved mer’s complex behavior in such a wide range of phenotypic variations. Annu. Rev. Cell Dev. Biol. 2008.24:501-523. Downloaded from www.annualreviews.org 1081-0706/08/1110-0501$20.00 Furthermore, the concept that endocytosis provides the space-time for signal transduction can now be experimentally addressed. In this review, we discuss these principles and argue for a systematic and top-down ap- proach to study the endocytic membrane system. We provide a summary of published observations on protein kinases regulating endocytic ma- chinery components and discuss global unbiased approaches to further map out kinase regulatory networks. -

Snareopathies: Diversity in Mechanisms and Symptoms
VU Research Portal SNAREopathies Verhage, Matthijs; Sørensen, Jakob B. published in Neuron 2020 DOI (link to publisher) 10.1016/j.neuron.2020.05.036 document version Publisher's PDF, also known as Version of record document license Article 25fa Dutch Copyright Act Link to publication in VU Research Portal citation for published version (APA) Verhage, M., & Sørensen, J. B. (2020). SNAREopathies: Diversity in Mechanisms and Symptoms. Neuron, 107(1), 22-37. https://doi.org/10.1016/j.neuron.2020.05.036 General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. E-mail address: [email protected] Download date: 29. Sep. 2021 ll Review SNAREopathies: Diversity in Mechanisms and Symptoms Matthijs Verhage1,2,* and Jakob B. Sørensen3,* 1Department of Functional Genomics, Vrije Universiteit (VU) Amsterdam, De Boelelaan 1085, Amsterdam 1081 HV, the Netherlands 2Department of Clinical Genetics, UMC Amsterdam, De Boelelaan 1085, Amsterdam 1081 HV, the Netherlands 3Department of Neuroscience, University of Copenhagen, 2200 Copenhagen N, Denmark *Correspondence: [email protected] (M.V.), [email protected] (J.B.S.) https://doi.org/10.1016/j.neuron.2020.05.036 Neuronal SNAREs and their key regulators together drive synaptic vesicle exocytosis and synaptic transmis- sion as a single integrated membrane fusion machine. -

Download Download
ISSN: 2469-6706 Vol. 6 2019 Role of epigenetics in the pathogenesis and management of type 2 diabetes mellitus Tajudeen Yahaya a , 1 Esther Oladele b, Ufuoma Shemishere c and Mohammad Abdulrau’f c aDepartment of Biology, Federal University Birnin Kebbi, Nigeria,bBiology Unit, Distance Learning Institute, University of Lagos, Nigeria, and cDepartment of Biochem- istry and Molecular Biology, Federal University Birnin Kebbi, Nigeria The need to reverse the growing incidence and burden of dia- ence the risk of T2DM, most of the candidate genes impair insulin betes mellitus (DM) worldwide has led to more studies on the synthesis rather than insulin metabolism (12, 13). This suggests that causes of the disease. Scientists have long suspected genetic pancreatic islet developmental error might be the main mechanism and environmental factors in the pathogenesis of type 2 diabetes of T2DM pathogenesis (12, 14). The suspect genes do not account mellitus (T2DM). However, recent studies suggest that epigenetic for the full transmission of T2DM, meaning that more genetic as- changes may cause some cases of the disease. This review high- lights the role of epigenetic modifications in the pathogenesis pect exists (12, 15). The search for this additional genetic factor led and management of T2DM. Peer-reviewed studies on the sub- to the discovery that modifications of the chemical tags above the ject were retrieved from electronic databases such as PubMed, genome, often known as epigenetic change, may modulate T2DM Google Scholar, SpringerLink, and Scopus. Most of the studies (1). implicated epigenetic modifications in the pathogenesis of some cases of T2DM. DNA methylation, histone modification, and mi- Epigenetic changes are heritable modifications in gene expres- croRNAs mediated pathways are the main mechanisms of epi- sion and function without affecting the nucleotide sequence (16, genetic changes. -

Perkinelmer Genomics to Request the Saliva Swab Collection Kit for Patients That Cannot Provide a Blood Sample As Whole Blood Is the Preferred Sample
Autism and Intellectual Disability TRIO Panel Test Code TR002 Test Summary This test analyzes 2429 genes that have been associated with Autism and Intellectual Disability and/or disorders associated with Autism and Intellectual Disability with the analysis being performed as a TRIO Turn-Around-Time (TAT)* 3 - 5 weeks Acceptable Sample Types Whole Blood (EDTA) (Preferred sample type) DNA, Isolated Dried Blood Spots Saliva Acceptable Billing Types Self (patient) Payment Institutional Billing Commercial Insurance Indications for Testing Comprehensive test for patients with intellectual disability or global developmental delays (Moeschler et al 2014 PMID: 25157020). Comprehensive test for individuals with multiple congenital anomalies (Miller et al. 2010 PMID 20466091). Patients with autism/autism spectrum disorders (ASDs). Suspected autosomal recessive condition due to close familial relations Previously negative karyotyping and/or chromosomal microarray results. Test Description This panel analyzes 2429 genes that have been associated with Autism and ID and/or disorders associated with Autism and ID. Both sequencing and deletion/duplication (CNV) analysis will be performed on the coding regions of all genes included (unless otherwise marked). All analysis is performed utilizing Next Generation Sequencing (NGS) technology. CNV analysis is designed to detect the majority of deletions and duplications of three exons or greater in size. Smaller CNV events may also be detected and reported, but additional follow-up testing is recommended if a smaller CNV is suspected. All variants are classified according to ACMG guidelines. Condition Description Autism Spectrum Disorder (ASD) refers to a group of developmental disabilities that are typically associated with challenges of varying severity in the areas of social interaction, communication, and repetitive/restricted behaviors. -

Oxidized Phospholipids Regulate Amino Acid Metabolism Through MTHFD2 to Facilitate Nucleotide Release in Endothelial Cells
ARTICLE DOI: 10.1038/s41467-018-04602-0 OPEN Oxidized phospholipids regulate amino acid metabolism through MTHFD2 to facilitate nucleotide release in endothelial cells Juliane Hitzel1,2, Eunjee Lee3,4, Yi Zhang 3,5,Sofia Iris Bibli2,6, Xiaogang Li7, Sven Zukunft 2,6, Beatrice Pflüger1,2, Jiong Hu2,6, Christoph Schürmann1,2, Andrea Estefania Vasconez1,2, James A. Oo1,2, Adelheid Kratzer8,9, Sandeep Kumar 10, Flávia Rezende1,2, Ivana Josipovic1,2, Dominique Thomas11, Hector Giral8,9, Yannick Schreiber12, Gerd Geisslinger11,12, Christian Fork1,2, Xia Yang13, Fragiska Sigala14, Casey E. Romanoski15, Jens Kroll7, Hanjoong Jo 10, Ulf Landmesser8,9,16, Aldons J. Lusis17, 1234567890():,; Dmitry Namgaladze18, Ingrid Fleming2,6, Matthias S. Leisegang1,2, Jun Zhu 3,4 & Ralf P. Brandes1,2 Oxidized phospholipids (oxPAPC) induce endothelial dysfunction and atherosclerosis. Here we show that oxPAPC induce a gene network regulating serine-glycine metabolism with the mitochondrial methylenetetrahydrofolate dehydrogenase/cyclohydrolase (MTHFD2) as a cau- sal regulator using integrative network modeling and Bayesian network analysis in human aortic endothelial cells. The cluster is activated in human plaque material and by atherogenic lipo- proteins isolated from plasma of patients with coronary artery disease (CAD). Single nucleotide polymorphisms (SNPs) within the MTHFD2-controlled cluster associate with CAD. The MTHFD2-controlled cluster redirects metabolism to glycine synthesis to replenish purine nucleotides. Since endothelial cells secrete purines in response to oxPAPC, the MTHFD2- controlled response maintains endothelial ATP. Accordingly, MTHFD2-dependent glycine synthesis is a prerequisite for angiogenesis. Thus, we propose that endothelial cells undergo MTHFD2-mediated reprogramming toward serine-glycine and mitochondrial one-carbon metabolism to compensate for the loss of ATP in response to oxPAPC during atherosclerosis. -

A Potential Target Gene of 9P13.3 Amplicon in Prostate Cancer
UNC13B – A potential target gene of 9p13.3 amplicon in prostate cancer Master´s thesis Anni Vesamäki University of Tampere BioMediTech May 2015 ACKNOWLEDGEMENTS The practical and written parts of this thesis were done in the Molecular Biology of Prostate Cancer Group, BioMediTech (BMT), University of Tampere. First, I would like to thank Professor Tapio Visakorpi for giving me the opportunity to work in his research group during several summers and during the practical work of this thesis. I would like to also thank all the members of the research group for their great advices and memorable times together. Especially, I would like thank my lovely supervisor PhD Leena Latonen for her quidance, experience and patience during this process. I also want to thank technicians Mariitta Vakkuri and Päivi Martikainen for their technical and practical help in the laboratory. Especially Mariitta, who´s memory will always stay in my heart. I would like to thank my family for all the support during these many years of studies. Especially my beloved husband who persistently pushed me forward and never stopped believing me, and my two gorgeous daughters who are the light of my life. Tampere, May 2015 Anni Vesamäki i PRO GRADU-TUTKIELMA Paikka: Tampereen yliopisto BioMediTech Tekijä: VESAMÄKI, ANNI MARIA Otsikko: UNC13B – A potential target gene of 9p13.3 amplicon in prostate cancer Sivut: 57 sivua, 1 liitesivu Ohjaajat: Professori Tapio Visakorpi ja FT Leena Latonen Tarkastajat: Professorit Markku Kulomaa ja Tapio Visakorpi Aika: Toukokuu 2015 ___________________________________________________________________________ TIIVISTELMÄ Tutkimuksen tausta ja tavoitteet: Eturauhassyöpä on miesten yleisin syöpä länsimaissa. Se on erittäin heterogeeninen sairaus niin kliinisesti kuin geneettisestikin.