Aroma Characteristics of Various' True'and'false'peppers from Around
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2017/0143022 A1 Wicker Et Al
US 20170143022A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0143022 A1 Wicker et al. (43) Pub. Date: May 25, 2017 (54) COMPOSITIONS INCORPORATING AN (52) U.S. Cl. UMLAM FLAVORAGENT CPC ............... A2.3L 27/20 (2016.08); A23L 27/88 (2016.08); A23L 2/56 (2013.01); A23L 2780 (71) Applicant: Senomyx, Inc., San Diego, CA (US) (2016.08); A23L 27/30 (2016.08); A23K 20/10 (2016.05); A23K 50/40 (2016.05); A61K 47/22 (72) Inventors: Sharon Wicker, Carlsbad, CA (US); (2013.01) Tanya Ditschun, San Diego, CA (US) (21) Appl. No.: 14/948,101 (57) ABSTRACT The present invention relates to compositions containing (22) Filed: Nov. 20, 2015 flavor or taste modifiers, such as a flavoring or flavoring agents and flavor or taste enhancers, more particularly, Publication Classification savory (“umami”) taste modifiers, savory flavoring agents (51) Int. Cl. and savory flavor enhancers, for foods, beverages, and other AOIN 25/00 (2006.01) comestible compositions. Compositions comprising an A23K2O/It (2006.01) umami flavor agent or umami taste-enhancing agent in A6 IK 47/22 (2006.01) combination with one or more other food additives, prefer A2.3L 2/56 (2006.01) ably including a flavorant, herb, Spice, fat, or oil, are A23K 50/40 (2006.01) disclosed. US 2017/O 143022 A1 May 25, 2017 COMPOSITIONS INCORPORATING AN tions WO 02/06254, WO 00/63166 art, WO 02/064631, and UMAM FLAVORAGENT WO 03/001876, and U.S. Patent publication US 2003 0232407 A1. The entire disclosures of the articles, patent BACKGROUND OF THE INVENTION applications, -

Piper Borbonense Mathieu Weil, Alain Shum Cheong Sing, Jean-Michel Méot, Renaud Boulanger, Philippe Bohuon
Impact of blanching, sweating and drying operations on pungency, aroma and color of Piper borbonense Mathieu Weil, Alain Shum Cheong Sing, Jean-Michel Méot, Renaud Boulanger, Philippe Bohuon To cite this version: Mathieu Weil, Alain Shum Cheong Sing, Jean-Michel Méot, Renaud Boulanger, Philippe Bohuon. Im- pact of blanching, sweating and drying operations on pungency, aroma and color of Piper borbonense. Food Chemistry, Elsevier, 2017, 219, pp.274 - 281. 10.1016/j.foodchem.2016.09.144. hal-01400687 HAL Id: hal-01400687 https://hal.univ-reunion.fr/hal-01400687 Submitted on 23 Nov 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Impact of blanching, sweating and drying operations on pungency, aroma and color of Piper borbonense a,⇑ b c c d M. Weil , A. Shum Cheong Sing , J.M. Méot , R. Boulanger , P. Bohuon a CIRAD – UMR Qualisud, Food Process Engineering Research Unit, 7 chemin de l’Irat, 97410 Saint Pierre – BP 180, Reunion b Université de la Réunion – Faculté des Sciences et Technologies Laboratoire de Chimie des Substances Naturelles et des Sciences des Aliments (LCSNSA), 15, avenue René Cassin, Moufia, Reunion c CIRAD, UMR QualiSud, Food Process Engineering Research Unit, TA B-95/16, 73°rue J.F. -
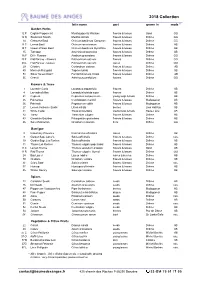
2018Maycollection Eng Maj19
2018 Collection latin name part grown in mode * Garden Herbs 12 P English Peppermint Mentha piperita Mitcham flowers & leaves Gard GG 12 N Spearmint Nanah Mentha spicata flowers & leaves Drôme GG 14 Genovese Basil Ocimum basilicum Genovese flowers & leaves Drôme GG 14 C Lemon Basil Ocimum americanum flowers & leaves Drôme AB 14 T Queen of Siam Basil Ocimum basilicum thyrsiflora flowers & leaves Drôme AB 15 Tarragon Artemisia dracunculus flowers & leaves Drôme AB 16 F Dill - Flowers Anethum graveolens flowers & leaves Drôme GG 20 F Flat Parsley - Flowers Petroselinum sativum flowers Drôme GG 20 L Flat Parsley - Leaves Petroselinum sativum leaves Drôme GG 29 Cilantro Coriandrum sativum flowers & leaves Drôme AB 40 Mexican Marygold Tagetes lucida flowers & leaves Drôme AB 53 Shiso "Green Kaori" Perilla frutescens crispa flowers & leaves Drôme AB 55 Chervil Anthriscus cerefolium flowers Drôme GG Flowers & Trees 1 Lavender Carla Lavandula angustifolia flowers Drôme AB 4 Lavendin d'Alba Lavandula hybrida super flowers Drôme AB 21 Cypress Cupressus sempervirens young twigs & buds Drôme AB 25 Palmarosa Cymbopogon martinii flowers & leaves Madagascar AB 26 Patchouli Pogostemon cablin flowers & leaves Madagascar AB 27 Lemon Verbena - Exotic Litsea citrata berries Laos Hat Kay AB 31 White Cedar Thuja occidentalis young twigs & buds Haute-Loire AB 42 Tansy Tanacetum vulgare flowers & leaves Drôme AB 43 Geranium Bourbon Pelargonium graveolens flowers & leaves Drôme AB 46 Sweet Marjoram Origanum majorana bells Drôme GG Garrigue 8 Rosemary Provence Rosmarinus officinalis leaves Drôme AB 9 Garden Sage Lamy's Salvia officinalis flowers & leaves Drôme rais. 10 Garden Sage Les Roches Salvia officinalis flowers & leaves Drôme AB 11 Thyme Les Roches Thymus vulgaris pop. -

PEPPER (Piper Nigrum) ABSTRACT
Journal of Food Technology Research 2021 Vol. 8, No. 1, pp. 18-25. ISSN(e): 2312-3796 ISSN(p): 2312-6426 DOI: 10.18488/journal.58.2021.81.18.25 © 2021 Conscientia Beam. All Rights Reserved. EFFECT OF BLANCHING TIME AND DRYING METHOD ON QUALITY OF BLACK PEPPER (Piper nigrum) 1,4,5,6,7 Suchana Paul1+ Scientific Officer, Bangladesh Agricultural Research Institute, Bangladesh. Rumman Ara2 Munshi Rashid (+ Corresponding author) Ahmad3 Pradip Hajong4 2Principal Scientific Officer, Bangladesh Agricultural Research Institute, 5 Gourango Paul Bangladesh. Md. Shahriar Kobir6 3Chief Scientific Officer, Bangladesh Agricultural Research Institute, Bangladesh. Md Hafizur Rahman7 ABSTRACT Article History Quality of black pepper highly depends on pre-drying treatment and drying method. A Received: 12 January 2021 pre-drying treatment, blanching (dipping in boiled water), is practiced in different Revised: 8 February 2021 Accepted: 26 February 2021 countries. However, Bangladesh is yet to follow this technique to produce black pepper. Published: 9 March 2021 So, this study investigated the effect of blanching, blanching time, and drying method on black pepper quality. Pepper berries were treated in boiled water (blanched) for 1, 2, Keywords 3, 4, 5 minutes and kept fresh (untreated). Both treated and untreated pepper berries Black pepper Processing were dried under the open sun and in a mechanical dryer. Results showed that Blanching blanching time negatively correlated with drying duration for both mechanical and sun- Blanching time Color drying method. Blanching limited the moisture content of dried pepper 5.33-11.52%, Drying where the moisture content of untreated sun-dried black pepper was more than 12%. -

Universidade Federal Do Acre Jamila Farias Mendonça Óleos Essenciais De Piperáceas Como Alternativas Para O Controle De Praga
UNIVERSIDADE FEDERAL DO ACRE JAMILA FARIAS MENDONÇA ÓLEOS ESSENCIAIS DE PIPERÁCEAS COMO ALTERNATIVAS PARA O CONTROLE DE PRAGAS RIO BRANCO ACRE - BRASIL JULHO - 2019 JAMILA FARIAS MENDONÇA ÓLEOS ESSENCIAIS DE PIPERÁCEAS COMO ALTERNATIVAS PARA O CONTROLE DE PRAGAS Dissertação apresentada à Universidade Federal do Acre, como parte das exigências do Programa de Pós- Graduação em Ciência Florestal, para a obtenção do título de Mestra em Ciência Florestal. Orientador: Prof. Dr. Adalberto Hipólito de Sousa. Coorientador: Prof. Dr. Carromberth Carioca Fernandes. RIO BRANCO ACRE – BRASIL JULHO – 2019 À minha mãe, motivo pelo qual me fez prosseguir. Ao meu pai Walter Pereira de Mendonça (in memoriam). E ao meu irmão Valcileudo Farias Mendonça (in memoriam). Dedico. AGRADECIMENTOS Agradeço ao meu Deus, por “tudo o que tenho; tudo o que sou; o que viera a ser devo a ti Senhor”. Sou grata à minha família, em especial à minha mãe, por todo auxílio durante esse período de mestrado. A meu pai Walter (in memoriam), que em vida foi um exemplo de ser humano. A meu irmão Valcileudo (mano) (in memoriam), que em vida (mesmo em silêncio) me ensinou o que significa persistência, força e fé. Ao Professor Dr. Adalberto Hipólito de Sousa, pela orientação durante esses anos, desde a graduação até o mestrado. Agradeço pela paciência e compreensão nos momentos difíceis e pelos ensinamentos repassados, estes me fizeram crescer como profissional. Tens minha admiração e gratidão. Ao Professor Dr. Carromberth Carioca Fernandes, por todo apoio durante o mestrado, pelos ensinamentos repassados e amizade. O destino nos uniu e despertou minha admiração pelo excelente profissional e ser humano que és. -

Die Kräuter Der Welt
12. Ausgabe 2017/2018 gültig ab Ende November 2016 DIE KRÄUTER DER WELT Kräuter und Gewürze, Tees, Tinkturen, ätherische und fette Öle, Fachliteratur, Drogeriewaren und vieles mehr... ® Inhaltsverzeichnis Seite Seite Auf einen Blick 3 Öle, Hydrolate, Räuchermittel Ätherische Öle 72 Unsere Website www.kraeuterschulte.de 4 Duftlampen und Duftsteine 80 Wichtige Hinweise - bitte unbedingt lesen 5 Hydrolate und Gesichtswässer 79 Öle (Speise-, Basis- und Körperöle) 80 Zentrales Kräuter- Parfümöle und Duftaromen 79 und Tinkturenregister Räucherkerzen, Räucherstäbchen 79 Pflanzliche Rohstoffe, Kräuter und Gewürze, Räucherkohle 79 aus konventionellem und biologischem Anbau sowie Wildsammlung 6 Klosterprodukte aus der Abtei St. Severin, Kaufbeuren 95 Aufbewahrung und Aufbereitung von Kräutern Aufbewahrung von Kräutern 48 Drogerie-Waren Kapselfüllgerät, Leerkapseln 48 Arzneikräuter in Fertigpackungen 84 Messzylinder 48 Arzneikräuter-Tropfen 86 Mörser & Pistill 49 Arzneimittel freiverkäuflich 86 Salbenkruken (Salbendosen) 49 Arzneisalben, Einreibungen, Augenpflege 93 Trichter 48 Bachblüten für Tiere 96 Tropf-, Medizin- und Pipettenflaschen 49 Bachblüten nach Dr. Bach 96 Weithalsgläser in vielen Größen 48 Badezusätze 99 Bergland Pflegeprodukte 99 Literatur Chanca Piedra Kapseln 97 Über Kräuter, Tinkturen, ätherische Öle etc. 50 Chinesische Nahrungsergänzungsmittel 97 Diät-Produkte, Reformwaren, Rohkost 83 Tees Einzelkräuter in Fertigpackungen 84 Kleine Teekunde 59 Erkältungspräparate 92 Ayurvedische Tees im Filterbeutel 84 Fußpflege 100 Ayurvedische -

Bogotá, D.C. 2011
UNIVERSIDAD DE LOS ANDES FACULTAD DE CIENCIAS DEPARTAMENTO DE CIENCIAS BIOLÓGICAS PIPER FOSSIL FROM A NEOTROPICAL FOREST OF THE LATE CRETACEOUS OF COLOMBIA : INFERRED AGES OF ORIGIN AND PATTERNS OF DIVERSIFICATION OF THE GENUS CAMILA MARTÍNEZ AGUILLÓN BOGOTÁ , D.C. 2011 UNIVERSIDAD DE LOS ANDES FACULTAD DE CIENCIAS DEPARTAMENTO DE CIENCIAS BIOLÓGICAS PIPER FOSSIL FROM A NEOTROPICAL FOREST OF THE LATE CRETACEOUS OF COLOMBIA : INFERRED AGES OF ORIGIN AND PATTERNS OF DIVERSIFICATION OF THE GENUS CAMILA MARTÍNEZ AGUILLÓN TESIS DE POSGRADO PARA OPTAR AL TÍTULO DE MAGÍSTER EN CIENCIAS BIOLÓGICAS DIRECTOR : SANTIAGO MADRIÑÁN (U NIANDES ) CODIRECTOR : CARLOS JARAMILLO (STRI) BOGOTÁ , D.C. 2011 ABSTRACT • Premise of study: The description of the new fossil Chavicoides schilleriphyllum from the late Cretaceous of Colombia is not only the first record associated with Piperaceae, but one of the oldest angiosperm macrofossils from the Neotropics. The study of C. schilleriphyllum within a phylogenetic framework allows an approach to understand the evolutionary history of Piper . • Methods: Leaf architecture characters of the fossil were compared to extant angiosperms. The phylogenetic position of C. schilleriphyllum was established based on a combined analysis of a molecular topology and a morphological matrix in order to make an internal calibration of the phylogeny of extant species. • Key Results: The dated phylogeny estimates that the genus Piper was originated in the Early Cretaceous. The diversification events started since the Late Cretaceous and continued gradually increasing until end of the Paleogene when the diversification rates turned exponential. • Conclusions : Chavicoides schilleriphyllum suggest that the habitat of Piper in the Late Cretaceous was an understory of a warm and wet forest. -

Reconstituted Plant Material and Its Use for Packaging
(19) TZZ¥_ __T (11) EP 3 122 941 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: D21H 27/10 (2006.01) B65D 65/02 (2006.01) 06.03.2019 Bulletin 2019/10 B65D 65/38 (2006.01) D21H 17/02 (2006.01) (21) Application number: 15713709.2 (86) International application number: PCT/EP2015/056745 (22) Date of filing: 27.03.2015 (87) International publication number: WO 2015/144893 (01.10.2015 Gazette 2015/39) (54) RECONSTITUTED PLANT MATERIAL AND ITS USE FOR PACKAGING, WRAPPING AND FOOD APPLIANCES REKONSTITUIERTES PFLANZENMATERIAL UND DESSEN VERWENDUNG FÜR VERPACKUNGS-, EINWICKEL- UND SPEISEVORRICHTUNGEN MATÉRIAU VÉGÉTAL RECONSTITUÉ ET SON UTILISATION À DES FINS D’EMPAQUETAGE, D’EMBALLAGE ET POUR DES DISPOSITIFS À USAGE ALIMENTAIRE (84) Designated Contracting States: • ROUSSEAU, Cédric AL AT BE BG CH CY CZ DE DK EE ES FI FR GB F-72000 Le Mans (FR) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • PONS, Esther PL PT RO RS SE SI SK SM TR F-33600 Pessac (FR) (30) Priority: 28.03.2014 US 201461971580 P (74) Representative: Jones Day Rechtsanwälte,Attorneys-at-Law, Patentanwälte (43) Date of publication of application: Prinzregentenstrasse 11 01.02.2017 Bulletin 2017/05 80538 München (DE) (73) Proprietor: SWM Luxembourg s.a.r.l. (56) References cited: 5326 Contern (LU) EP-A1- 0 500 931 EP-A1- 0 630 828 WO-A1-2011/094840 JP-A- 2001 104 153 (72) Inventors: US-A- 3 847 164 US-A- 3 860 012 • RAGOT, Philippe US-A- 4 182 349 US-A- 5 006 405 LU-5720 Aspelt (LU) US-A- 5 858 487 US-A1- 2003 079 846 • BARAT, Laetitia F-72100 Le Mans (FR) Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

(12) Patent Application Publication (10) Pub. No.: US 2017/0096418 A1 Patron Et Al
US 201700 96418A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0096418 A1 Patron et al. (43) Pub. Date: Apr. 6, 2017 (54) COMPOUNDS USEFUL AS MODULATORS Related U.S. Application Data OF TRPM8 (60) Provisional application No. 62/236,080, filed on Oct. (71) Applicant: Senomyx, Inc., San Diego, CA (US) 1, 2015. (72) Inventors: Andrew P. Patron, San Marcos, CA Publication Classification (US); Alain Noncovich, San Diego, CA (51) Int. Cl. (US); Jane Ung, San Diego, CA (US); C07D 409/2 (2006.01) Timothy Davis, Santee, CA (US); A6IR 9/00 (2006.01) Joseph R. Fotsing, San Diego, CA A63L/455 (2006.01) (US); Chad Priest, Leucadia, CA (US); (52) U.S. Cl. Catherine Tachdjian, San Diego, CA CPC ........ C07D 409/12 (2013.01); A61K 31/4155 (US) (2013.01); A61K 9/0014 (2013.01) (21) Appl. No.: 15/282,432 (57) ABSTRACT The present disclosure relates to compounds which are (22) Filed: Sep. 30, 2016 useful as cooling sensation compounds. US 2017/0096418 A1 Apr. 6, 2017 COMPOUNDS USEFUL AS MODULATORS -continued OF TRPM8 RELATED APPLICATIONS 0001. This application claims the benefit of U.S. Provi sional Application No. 62/236,080, filed Oct. 1, 2015, which is incorporated herein by reference in its entirety. BACKGROUND 0002 Field 0003. The present invention relates to compounds useful as cooling agents. 0004 Background Description 0005 Modulators of the Melastatin Transient Receptor or a salt or solvate thereof. Potential Channel 8 (TRPM8) are known to have a cooling effect. TRPM8 is a channel involved in the chemesthetic O008) In some embodiments the compound can have the sensation, such as cool to cold temperatures as well as the Structure sensation of known cooling agents. -

Piper Africains Et De L’OI
Séminaire « Rencontres de l’Agroalimentaire en Océan Indien QualiREG 2016 - 5ème édition Le Tsiperifery ou poivre sauvage ou à queue de Madagascar diffère des autres Piper africains et de l’OI Hanitra ANDRIANOELISOA, Jean Michel LEONG POCK TSY, Harizoly RAZAFIMANDIMBY Mathieu WEIL, Anne Gaëlle BENARD, Jérôme QUESTE, Lolona RAMAMONJISOA, Pascal DANTHU Objectif Mettre en évidence l’existence de grandes variabilités morphologique, génétique et chimique au sein du Tsiperifery de Madagascar et des autres Piper africains et de La Réunion Produits forestiers non-ligneux de Madagcascar : cas du Tsiperifery Le poivre sauvage de Madagascar, ou Tsiperifery, est une épice de luxe commercialisée en Europe depuis 2010 sur un marché de niche à forte valeur ajoutée. Prix FOB: 15 à 25€ selon la qualité. La filière est émergente mais très (trop?) dynamique. Sa liane fructifie dans la canopée, ce qui amène les cueilleurs à abattre les tuteurs. => Exploitation detructive 3 Méthodologies appliquées Tsiperifery est présent du Nord au Sud dans les forêts sempervirentes de l’Est. Sa cueillette profite aux membres les plus pauvres des communautés Sites d’études ~ le bassin d’exploitation d’Anjozorobe ~ les stations de recherche du FOFIFA de Beforona et de Sandrangato -Tsiazompaniry -Anosibe Anala Méthodologies appliquées Diversité morphologique Morphométrie basée sur des critères botaniques Analyse factorielle de données mixtes (AFDM) avec le logiciel R. Diversité génétique Analyse comparative des séquences d’un gène chloroplastique et d’un gène nucléaire (marqueurs publiés par JF Smith et al. 2008 et MA Jaramillo et al. 2008) Diversité Chimique Distillation CPG, CPG-SM Classification des données chimiques des HE Résultats Diversité morphologie : 4 morphotypes Grosses baies Petites baies Résultats Diversité génétique : 2 groupes petites baies Morphotypes à petits grains (M2 et M3) Morphotypes à gros grains (M1 et M4) grosses baies Piper nigrum Piper guineense Piper capense Piper borbonense Tsiperifery Poivres à queue P. -

Comparison of Traditional and Novel Drying Techniques and Its Effect On
foods Review Comparison of Traditional and Novel Drying Techniques and Its Effect on Quality of Fruits, Vegetables and Aromatic Herbs Ángel Calín-Sánchez 1,* , Leontina Lipan 1 , Marina Cano-Lamadrid 1 , Abdolreza Kharaghani 2 , Klaudia Masztalerz 3 , Ángel A. Carbonell-Barrachina 1 and Adam Figiel 3 1 Agrofood Technology Department, Universidad Miguel Hernández de Elche, 03312 Orihuela, Spain; [email protected] (L.L.); [email protected] (M.C.-L.); [email protected] (Á.A.C.-B.) 2 Thermal Process Engineering, Otto von Guericke University, P.O. 4120, 39016 Magdeburg, Germany; [email protected] 3 Institute of Agricultural Engineering, Wrocław University of Environmental and Life Sciences, P.O. 37/41, 51-630 Wrocław, Poland; [email protected] (K.M.); adam.fi[email protected] (A.F.) * Correspondence: [email protected]; Tel.: +34-966-749-738 Received: 3 August 2020; Accepted: 4 September 2020; Published: 9 September 2020 Abstract: Drying is known as the best method to preserve fruits, vegetables, and herbs, decreasing not only the raw material volume but also its weight. This results in cheaper transportation and increments the product shelf life, limiting the food waste. Drying involves the application of energy in order to vaporize and mobilize the moisture content within the porous products. During this process, the heat and mass transfer occurs simultaneously. The quality of dehydrated fruits, vegetables, and aromatic herbs is a key problem closely related to the development and optimization of novel drying techniques. This review reports the weaknesses of common drying methods applied for fruits, vegetables, and aromatic herbs and the possible options to improve the quality of dried products using different drying techniques or their combination. -

The Roots—An Overview of the Chemical Composition and Bioactivity of Selected Root-Essential Oils
molecules Review Back to the Roots—An Overview of the Chemical Composition and Bioactivity of Selected Root-Essential Oils Karin Lunz and Iris Stappen * Department of Pharmaceutical Sciences, University of Vienna, 1090 Vienna, Austria; [email protected] * Correspondence: [email protected]; Tel.: +43-1-4277-55552 Abstract: Since ancient times, plant roots have been widely used in traditional medicine for treating various ailments and diseases due to their beneficial effects. A large number of studies have demon- strated that—besides their aromatic properties—their biological activity can often be attributed to volatile constituents. This review provides a comprehensive overview of investigations into the chemical composition of essential oils and volatile components obtained from selected aromatic roots, including Angelica archangelica, Armoracia rusticana, Carlina sp., Chrysopogon zizanioides, Coleus forskohlii, Inula helenium, Sassafras albidum, Saussurea costus, and Valeriana officinalis. Additionally, their most important associated biological impacts are reported, such as anticarcinogenic, antimicro- bial, antioxidant, pesticidal, and other miscellaneous properties. Various literature and electronic databases—including PubMed, ScienceDirect, Springer, Scopus, Google Scholar, and Wiley—were screened and data was obtained accordingly. The results indicate the promising properties of root-essential oils and their potential as a source for natural biologically active products for flavor, pharmaceutical, agricultural, and fragrance industries. However, more research is required to further establish the mechanism of action mediating these bioactivities as well as essential oil standardization Citation: Lunz, K.; Stappen, I. Back because the chemical composition often strongly varies depending on external factors. to the Roots—An Overview of the Chemical Composition and Bioactivity Keywords: Angelica archangelica; Armoracia rusticana; Carlina sp.; Chrysopogon zizanioides; Coleus of Selected Root-Essential Oils.