Chemical Composition, Antioxidant and Antimicrobial Activity of Essential Oils Isolated from Two Piper Species Collected in Comoros
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Volatiles of Black Pepper Fruits (Piper Nigrum L.)
molecules Article Volatiles of Black Pepper Fruits (Piper nigrum L.) Noura S. Dosoky 1 , Prabodh Satyal 1, Luccas M. Barata 2 , Joyce Kelly R. da Silva 2 and William N. Setzer 1,3,* 1 Aromatic Plant Research Center, Suite 100, Lehi, UT 84043, USA; [email protected] (N.S.D.); [email protected] (P.S.) 2 Programa de Pós-Graduação em Biotecnologia, Universidade Federal do Pará, Belém 66075-110, PA, Brazil; [email protected] (L.M.B.); [email protected] (J.K.R.d.S.) 3 Department of Chemistry, University of Alabama in Huntsville, Huntsville, AL 35899, USA * Correspondence: [email protected]; Tel.: +1-256-824-6519 Academic Editor: Francesca Mancianti Received: 4 October 2019; Accepted: 5 November 2019; Published: 21 November 2019 Abstract: Black pepper (Piper nigrum) is historically one of the most important spices and herbal medicines, and is now cultivated in tropical regions worldwide. The essential oil of black pepper fruits has shown a myriad of biological activities and is a commercially important commodity. In this work, five black pepper essential oils from eastern coastal region of Madagascar and six black pepper essential oils from the Amazon region of Brazil were obtained by hydrodistillation and analyzed by gas chromatography-mass spectrometry. The major components of the essential oils were α-pinene, sabinene, β-pinene, δ-3-carene, limonene, and β-caryophyllene. A comparison of the Madagascar and Brazilian essential oils with black pepper essential oils from various geographical regions reported in the literature was carried out. A hierarchical cluster analysis using the data obtained in this study and those reported in the literature revealed four clearly defined clusters based on the relative concentrations of the major components. -

Trees and Plants for Bees and Beekeepers in the Upper Mara Basin
Trees and plants for bees and beekeepers in the Upper Mara Basin Guide to useful melliferous trees and crops for beekeepers December 2017 Contents Who is this guide for? .......................................................................................................................................................................................................................................................................... 1 Introduction to the MaMaSe Project .................................................................................................................................................................................................................................................. 1 Market driven forest conservation initiatives in the Upper Mara basin ............................................................................................................................................................................................. 2 Water, apiculture, forests, trees and livelihoods ................................................................................................................................................................................................................................ 3 Types of bees ....................................................................................................................................................................................................................................................................................... 4 How this -

(12) Patent Application Publication (10) Pub. No.: US 2017/0143022 A1 Wicker Et Al
US 20170143022A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0143022 A1 Wicker et al. (43) Pub. Date: May 25, 2017 (54) COMPOSITIONS INCORPORATING AN (52) U.S. Cl. UMLAM FLAVORAGENT CPC ............... A2.3L 27/20 (2016.08); A23L 27/88 (2016.08); A23L 2/56 (2013.01); A23L 2780 (71) Applicant: Senomyx, Inc., San Diego, CA (US) (2016.08); A23L 27/30 (2016.08); A23K 20/10 (2016.05); A23K 50/40 (2016.05); A61K 47/22 (72) Inventors: Sharon Wicker, Carlsbad, CA (US); (2013.01) Tanya Ditschun, San Diego, CA (US) (21) Appl. No.: 14/948,101 (57) ABSTRACT The present invention relates to compositions containing (22) Filed: Nov. 20, 2015 flavor or taste modifiers, such as a flavoring or flavoring agents and flavor or taste enhancers, more particularly, Publication Classification savory (“umami”) taste modifiers, savory flavoring agents (51) Int. Cl. and savory flavor enhancers, for foods, beverages, and other AOIN 25/00 (2006.01) comestible compositions. Compositions comprising an A23K2O/It (2006.01) umami flavor agent or umami taste-enhancing agent in A6 IK 47/22 (2006.01) combination with one or more other food additives, prefer A2.3L 2/56 (2006.01) ably including a flavorant, herb, Spice, fat, or oil, are A23K 50/40 (2006.01) disclosed. US 2017/O 143022 A1 May 25, 2017 COMPOSITIONS INCORPORATING AN tions WO 02/06254, WO 00/63166 art, WO 02/064631, and UMAM FLAVORAGENT WO 03/001876, and U.S. Patent publication US 2003 0232407 A1. The entire disclosures of the articles, patent BACKGROUND OF THE INVENTION applications, -

“Pimienta” De Los Géneros Piper, Pimenta, Lindera, Ruta, Schin
NOTAS BREVES Botanica Complutensis ISSN-e: 1988-2874 https://dx.doi.org/10.5209/bocm.73020 Composición de aceites esenciales de diferentes especies de “pimienta” de los géneros Piper, Pimenta, Lindera, Ruta, Schinus y Zanthoxylum Héctor Alonso-Miguel1, María José Pérez-Alonso1, Ana Cristina Soria2, Manuel Blanco Martínez1 Resumen. Se ha extraído mediante hidrodestilación el aceite esencial de diez especies usadas como pimienta: Piper borbonense, P. capense, P. retrofractum, P. nigrum, Zanthoxylum bungeanum y Z. armatum, Lindera neesiana, Ruta chalepensis, Schinus terebenthifolia, Pimenta dioica. Los análisis realizados mediante cromatografía de gases acoplada a espectrometría de masas encontraron que todas presentan β-felandreno y derivados de cariofileno y felandreno, siendo estos compuestos de propiedades pungentes los característicos de la especia pimienta. El rendimiento de esencia varía desde 0,43% para R. chalepensis hasta 7,61% para P. borbonense. Los compuestos mayoritarios fueron: P. borbonense (α-felandreno, 12,43%), P. capense (δ-cadineno, 25,59%,), P. retrofractum (γ-cadineno, 31,63%), P. nigrum ((E)-β-cariofileno, 22,88%), P. dioica (eugenol, 48,93%), L. neesiana (miristicina, 14,13%), R. chalepensis (2-undecanona, 64,93%), S. therebenthifolia (δ-3-careno, 29,21%), Z. armatum (linalool, 53,30%); Z. bungeanum (linalool, 64,09%). Todo esto muestra las diferencias en el metabolismo secundario de las pimientas y por tanto sus posibles aplicaciones en diferentes industrias. Palabras clave: Pimienta; especia; aceites esenciales; Piper; Ruta; Pimenta; Lindera; Schinus; Zanthoxylum [en] Composition of essential oils of different species of “pepper” of the Piper, Pimenta, Lindera, Ruta, Schinus and Zanthoxylum genera Abstract.The essential oil of ten species used as pepper has been extracted by hydrodistillation: Piper borbonense, P. -

Piper Borbonense Mathieu Weil, Alain Shum Cheong Sing, Jean-Michel Méot, Renaud Boulanger, Philippe Bohuon
Impact of blanching, sweating and drying operations on pungency, aroma and color of Piper borbonense Mathieu Weil, Alain Shum Cheong Sing, Jean-Michel Méot, Renaud Boulanger, Philippe Bohuon To cite this version: Mathieu Weil, Alain Shum Cheong Sing, Jean-Michel Méot, Renaud Boulanger, Philippe Bohuon. Im- pact of blanching, sweating and drying operations on pungency, aroma and color of Piper borbonense. Food Chemistry, Elsevier, 2017, 219, pp.274 - 281. 10.1016/j.foodchem.2016.09.144. hal-01400687 HAL Id: hal-01400687 https://hal.univ-reunion.fr/hal-01400687 Submitted on 23 Nov 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Impact of blanching, sweating and drying operations on pungency, aroma and color of Piper borbonense a,⇑ b c c d M. Weil , A. Shum Cheong Sing , J.M. Méot , R. Boulanger , P. Bohuon a CIRAD – UMR Qualisud, Food Process Engineering Research Unit, 7 chemin de l’Irat, 97410 Saint Pierre – BP 180, Reunion b Université de la Réunion – Faculté des Sciences et Technologies Laboratoire de Chimie des Substances Naturelles et des Sciences des Aliments (LCSNSA), 15, avenue René Cassin, Moufia, Reunion c CIRAD, UMR QualiSud, Food Process Engineering Research Unit, TA B-95/16, 73°rue J.F. -
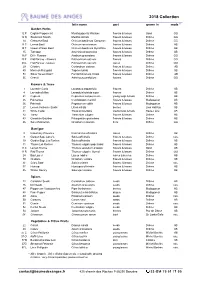
2018Maycollection Eng Maj19
2018 Collection latin name part grown in mode * Garden Herbs 12 P English Peppermint Mentha piperita Mitcham flowers & leaves Gard GG 12 N Spearmint Nanah Mentha spicata flowers & leaves Drôme GG 14 Genovese Basil Ocimum basilicum Genovese flowers & leaves Drôme GG 14 C Lemon Basil Ocimum americanum flowers & leaves Drôme AB 14 T Queen of Siam Basil Ocimum basilicum thyrsiflora flowers & leaves Drôme AB 15 Tarragon Artemisia dracunculus flowers & leaves Drôme AB 16 F Dill - Flowers Anethum graveolens flowers & leaves Drôme GG 20 F Flat Parsley - Flowers Petroselinum sativum flowers Drôme GG 20 L Flat Parsley - Leaves Petroselinum sativum leaves Drôme GG 29 Cilantro Coriandrum sativum flowers & leaves Drôme AB 40 Mexican Marygold Tagetes lucida flowers & leaves Drôme AB 53 Shiso "Green Kaori" Perilla frutescens crispa flowers & leaves Drôme AB 55 Chervil Anthriscus cerefolium flowers Drôme GG Flowers & Trees 1 Lavender Carla Lavandula angustifolia flowers Drôme AB 4 Lavendin d'Alba Lavandula hybrida super flowers Drôme AB 21 Cypress Cupressus sempervirens young twigs & buds Drôme AB 25 Palmarosa Cymbopogon martinii flowers & leaves Madagascar AB 26 Patchouli Pogostemon cablin flowers & leaves Madagascar AB 27 Lemon Verbena - Exotic Litsea citrata berries Laos Hat Kay AB 31 White Cedar Thuja occidentalis young twigs & buds Haute-Loire AB 42 Tansy Tanacetum vulgare flowers & leaves Drôme AB 43 Geranium Bourbon Pelargonium graveolens flowers & leaves Drôme AB 46 Sweet Marjoram Origanum majorana bells Drôme GG Garrigue 8 Rosemary Provence Rosmarinus officinalis leaves Drôme AB 9 Garden Sage Lamy's Salvia officinalis flowers & leaves Drôme rais. 10 Garden Sage Les Roches Salvia officinalis flowers & leaves Drôme AB 11 Thyme Les Roches Thymus vulgaris pop. -
Aroma Characteristics of Various' True'and'false'peppers from Around
COPYRIGHT AND USE OF THIS THESIS This thesis must be used in accordance with the provisions of the Copyright Act 1968. Reproduction of material protected by copyright may be an infringement of copyright and copyright owners may be entitled to take legal action against persons who infringe their copyright. Section 51 (2) of the Copyright Act permits an authorized officer of a university library or archives to provide a copy (by communication or otherwise) of an unpublished thesis kept in the library or archives, to a person who satisfies the authorized officer that he or she requires the reproduction for the purposes of research or study. The Copyright Act grants the creator of a work a number of moral rights, specifically the right of attribution, the right against false attribution and the right of integrity. You may infringe the author’s moral rights if you: - fail to acknowledge the author of this thesis if you quote sections from the work - attribute this thesis to another author - subject this thesis to derogatory treatment which may prejudice the author’s reputation For further information contact the University’s Director of Copyright Services sydney.edu.au/copyright The University of Sydney Aroma Characterisation of various ‘true’ and ‘false’ peppers from around the globe Hayden Druce Masters of Philosophy (Agriculture) Department of Plant and Food Science Faculty of Agriculture and Environment University of Sydney NSW, Australia 2013 i STATEMENT OF ORIGINALITY Except references cited, this thesis is the result of research undertaken for the completion of the Masters of Philosophy (Agriculture) at the University of Sydney. -

A 17 Year Successional Enrichment Plantation of Tree Recruitment and Restoration in an African Tropical Forest
A 17 Year Successional Enrichment Plantation of Tree Recruitment and Restoration in an African Tropical Forest Bernard Eromosele Omomoh ( [email protected] ) Federal University of Technology Akure School of Agriculture and Agricultural Technology https://orcid.org/0000-0002-0506-4973 Gbenga Festus Akomolafe Federal University of Laa, Department of Plant Science & Biotechnology, Laa, Nigeria Leah Spencer Brown Western University, Faculty of Information and Media Studies, London, Ontario, Canada VAJ Adekunle Forestry and Wood Tech, Federal University of Technology, Akure, Nigeria Research Article Keywords: aboveground tree, successional forest, disturbed forest, forest regeneration, sapling recruitment, post-disturbance Posted Date: April 7th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-138855/v2 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License A 17 Year Successional Enrichment Plantation of Tree Recruitment and Restoration in an African Tropical Forest 1 Abstract 2 Key message: The Enrichment Plantation of Akure Forest Reserve is one of the forests 3 currently experiencing a 17-year-long post-disturbance following deforestation and 4 fragmentation in Nigeria. 5 Context: To better understand the contribution of enrichment planting on forest regeneration 6 and restoration, when the Enrichment Plantation after 17 years of post-disturbance was 7 examined. 8 Aims: We studied the recruitment drive of aboveground and undergrowth stands of an 9 Enrichment Plantation in the tropical forest reserve. We assess the trees diversity, species 10 compositions, species richness, and growth forms of the vegetations. 11 Methods: A total of 3(50m x50m) plots were sampled. A total of 47 aboveground tree 12 species and 45 undergrowth stands from Enrichment Plantation were identified. -

Cover-Tab. Con-Abst-Declar, Final Version
ADDIS ABABA UNIVERSITY SCHOOL OF GRADUATE STUDIES HOMEGARDENS AND SPICES OF BASKETO AND KAFA (SOUTHWEST ETHIOPIA): PLANT DIVERSITY, PRODUCT VALORIZATION AND IMPLICATIONS TO BIODIVERSITY CONSERVATION By Feleke Woldeyes A Thesis Submitted to the School of Graduate Studies of Addis Ababa University in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Biology (Botanical Sciences) February 2011 Addis Ababa ADDIS ABABA UNIVERSITY SCHOOL OF GRADUATE STUDIES HOMEGARDENS AND SPICES OF BASKETO AND KAFA (SOUTHWEST ETHIOPIA): PLANT DIVERSITY, PRODUCT VALORIZATION AND IMPLICATIONS TO BIODIVERSITY CONSERVATION By Feleke Woldeyes A Thesis Submitted to the School of Graduate Studies of Addis Ababa University in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Biology (Botanical Sciences) Approved by the Examining Board: Prof. Sileshi Nemomissa (Internal Examiner) ________________________________ Prof. P. Van Damme (External Examiner) ________________________________ Dr. Zemede Asfaw (Supervisor) ________________________________ Prof. Sebsebe Demissew (Supervisor) ________________________________ Prof. Bernard Roussel (Supervisor) ________________________________ Prof. Zerihun Woldu (Chairman) ________________________________ DEDICATION This thesis is dedicated to the Basket and Kafecho peoples who, through innovative agricultural practices for generations, developed such a sustainable crop production system - Homegardening. ACKNOWLEDGEMENTS This is a work which became a reality through -

S. Mohana Roopan · G. Madhumitha Editors Bioorganic Phase in Natural Food: an Overview Bioorganic Phase in Natural Food: an Overview S
S. Mohana Roopan · G. Madhumitha Editors Bioorganic Phase in Natural Food: An Overview Bioorganic Phase in Natural Food: An Overview S. Mohana Roopan • G. Madhumitha Editors Bioorganic Phase in Natural Food: An Overview Editors S. Mohana Roopan G. Madhumitha Department of Chemistry Department of Chemistry Chemistry of Heterocycles & Natural Chemistry of Heterocycles & Natural Product Research Laboratory Product Research Laboratory School of Advanced Sciences School of Advanced Sciences Vellore Institute of Technology Vellore Institute of Technology Vellore, Tamil Nadu, India Vellore, Tamil Nadu, India ISBN 978-3-319-74209-0 ISBN 978-3-319-74210-6 (eBook) https://doi.org/10.1007/978-3-319-74210-6 Library of Congress Control Number: 2018935134 © Springer International Publishing AG, part of Springer Nature 2018 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. The publisher, the authors and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. -

(Ntfp) in Liberia
AN ENVIRONMENTAL AND ECONOMIC APPROACH TO THE DEVELOPMENT AND SUSTAINABLE EXPLOITATION OF NON-TIMBER FOREST PRODUCTS (NTFP) IN LIBERIA By LARRY CLARENCE HWANG A dissertation submitted to the Graduate School-New Brunswick Rutgers, The State University of New Jersey In partial fulfillment of the requirements For the degree of Doctor of Philosophy Graduate Program in Plant Biology Written under the direction of James E. Simon And approved by _________________________________________________ _________________________________________________ _________________________________________________ _________________________________________________ New Brunswick, New Jersey October 2017 ABSTRACT OF THE DISSERTATION An Environmental and Economic Approach to the Development and Sustainable Exploitation of Non-Timber Forest Products (NTFP) in Liberia by LARRY C. HWANG Dissertation Director: James E. Simon Forests have historically contributed immensely to influence patterns of social, economic, and environmental development, supporting livelihoods, aiding construction of economic change, and encouraging sustainable growth. The use of NTFP for the livelihood and subsistence of forest community dwellers have long existed in Liberia; with use, collection, and local/regional trade in NTFP still an ongoing activities of rural communities. This study aimed to investigate the environmental and economic approaches that lead to the sustainable management exploitation and development of NTFP in Liberia. Using household information from different socio-economic societies, knowledge based NTFP socioeconomics population, as well as abundance and usefulness of the resources were obtained through the use of ethnobotanical survey on use of NTFP in 82 rural communities within seven counties in Liberia. 1,165 survey participants, with 114 plant species listed as valuable NTFP. The socioeconomic characteristics of 255 local community people provided collection practice information on NTFP, impact and threats due to collection, and their income generation. -

PEPPER (Piper Nigrum) ABSTRACT
Journal of Food Technology Research 2021 Vol. 8, No. 1, pp. 18-25. ISSN(e): 2312-3796 ISSN(p): 2312-6426 DOI: 10.18488/journal.58.2021.81.18.25 © 2021 Conscientia Beam. All Rights Reserved. EFFECT OF BLANCHING TIME AND DRYING METHOD ON QUALITY OF BLACK PEPPER (Piper nigrum) 1,4,5,6,7 Suchana Paul1+ Scientific Officer, Bangladesh Agricultural Research Institute, Bangladesh. Rumman Ara2 Munshi Rashid (+ Corresponding author) Ahmad3 Pradip Hajong4 2Principal Scientific Officer, Bangladesh Agricultural Research Institute, 5 Gourango Paul Bangladesh. Md. Shahriar Kobir6 3Chief Scientific Officer, Bangladesh Agricultural Research Institute, Bangladesh. Md Hafizur Rahman7 ABSTRACT Article History Quality of black pepper highly depends on pre-drying treatment and drying method. A Received: 12 January 2021 pre-drying treatment, blanching (dipping in boiled water), is practiced in different Revised: 8 February 2021 Accepted: 26 February 2021 countries. However, Bangladesh is yet to follow this technique to produce black pepper. Published: 9 March 2021 So, this study investigated the effect of blanching, blanching time, and drying method on black pepper quality. Pepper berries were treated in boiled water (blanched) for 1, 2, Keywords 3, 4, 5 minutes and kept fresh (untreated). Both treated and untreated pepper berries Black pepper Processing were dried under the open sun and in a mechanical dryer. Results showed that Blanching blanching time negatively correlated with drying duration for both mechanical and sun- Blanching time Color drying method. Blanching limited the moisture content of dried pepper 5.33-11.52%, Drying where the moisture content of untreated sun-dried black pepper was more than 12%.