Analysis and Optimization of a Dissolved Air Flotation Process for Separation of Suspended Solids in Wastewater
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Operational Programme Under the 'Investment For
OPERATIONAL PROGRAMME UNDER THE ‘INVESTMENT FOR GROWTH AND JOBS’ GOAL CCI 2014SE16RFOP005 Title Stockholm Version 1.3 First year 2014 Last year 2020 Eligible from 01-Jan-2014 Eligible until 31-Dec-2023 EC decision number C(2014)9970 EC decision date 16-Dec-2014 MS amending decision number MS amending decision date MS amending decision entry into force date NUTS regions covered by SE110 — Stockholm County the operational programme EN EN EN 1. STRATEGY FOR THE OPERATIONAL PROGRAMME’S CONTRIBUTION TO THE UNION STRATEGY FOR SMART, SUSTAINABLE AND INCLUSIVE GROWTH AND THE ACHIEVEMENT OF ECONOMIC, SOCIAL AND TERRITORIAL COHESION 1.1 Strategy for the operational programme’s contribution to the Union strategy for smart, sustainable and inclusive growth and to the achievement of economic, social and territorial cohesion 1.1.1 Description of the programme’s strategy for contributing to the delivery of the Union strategy for smart, sustainable and inclusive growth and for achieving economic, social and territorial cohesion. The regional Structural Funds programme covers Stockholm County, which coincides with the geographical area of Stockholm in the European Union’s NUTS2 classification. Today, in 2013, the region has a population of just over 2.1 million, divided between 26 municipalities. The largest municipality, Stockholm City, in addition to being the national capital, is the largest municipality in the region (and in Sweden), with nearly 900 000 inhabitants. The smallest municipalities in the county, by comparison, have a population of around 10 000. The annual increase in population during the programming period 2007-2013 was just over 35 000. -
Read Full Report (PDF)
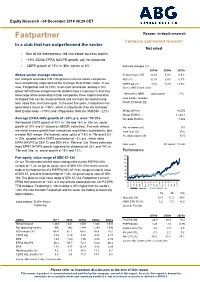
Equity Research - 04 December 2019 06:29 CET Fastpartner Reason: In-depth research Company sponsored research In a club that has outperformed the sector Not rated One of the entrepreneur-led real estate success stories ~19% 2020e EPRA NAVPS growth, adj. for dividends CEPS growth of 15% in ‘20e, sector at 6% Estimate changes (%) 2019e 2020e 2021e Above-sector average returns Rental income RE (%) 0.9% 5.9% 6.5% Our analysis concludes that entrepreneur-led real estate companies NOI (%) -0.1% 5.9% 6.7% have consistently outperformed the Carnegie Real Estate Index. In our CEPS adj (%) 3.7% 8.3% 11.5% view, Fastpartner and its CEO, Sven-Olof Johansson, belong in this Source: ABG Sundal Collier group. What these entrepreneurial leaders have in common is that they Share price (SEK) 02/12/2019 91.6 have large share ownership in their companies, have capital allocation strategies that can be unconventional and are more focused on long- Real Estate, Sweden term value than short-term gain. In the past five years, Fastpartner has FPAR.ST/FPAR SS generated a return of ~190%, which is 20pp better than the Carnegie Real Estate Index ~170%) and 170pp better than the OMXS30 ~22%). MCap (SEKm) 16,570 MCap (EURm) 1,568.7 Average EPRA NAV growth of ~20% p.a. over ’19-’21e Net debt (EURm) 1,269 We forecast CEPS growth of 17% in ’19e and 15% in ’20e (vs. sector growth of 10% and 6% based on ABGSC estimates). The main drivers No. of shares (m) 181 are rental income growth from announced acquisitions and projects, plus Free float (%) 30.0 a higher NOI margin. -
![“Yoake [夜明け]” a Gunslinger Girl Original Story by Kiskaloo](https://docslib.b-cdn.net/cover/3339/yoake-a-gunslinger-girl-original-story-by-kiskaloo-1063339.webp)
“Yoake [夜明け]” a Gunslinger Girl Original Story by Kiskaloo
This story uses characters and locations based on the Gunslinger Girl manga written by Yu Aida and published in monthly shōnen magazine Dengeki Daioh. The characters of Kara and Michele are original to myself. “Yoake [夜明け]” A Gunslinger Girl Original Story by Kiskaloo “Heaven is not enough…if when I am there I don’t remember you.” – Yoko Kanno. On a warm March Wednesday, in the passenger seat of a Lamborghini Gallardo Spyder parked on the side of the Autostrada 24 in a suburb east of Rome, Fleda Claes Johansson started to cry, her sobs drowned out by the flow of late afternoon traffic though Michele could see her chest heave and see the tears stream down her face. He removed a clean handkerchief from his pants pocket and handed it to Claes, who turned towards him with eyes red from tears and cheeks red from embarrassment. “Gratzie,” she said as she dabbed at her eyes and wiped her cheeks. She sniffled with a sharp intake of breath through her nose and her face set in a hard look, only a moment later to collapse into a fresh bout of crying. She buried her face in the handkerchief and willed herself into composure. “Crying like a schoolgirl rejected by her crush,” she said, her tone harsh. “You were conditioned to love him. And now that you remember him, it’s natural to miss him knowing that’s he is gone,” Michele offered. “What we shared wasn’t really love,” Claes noted. “I mean I did love him because the conditioning made me, but it made him uncomfortable.” She took in a few deep breaths to steady herself. -

To Reuse Or to Incinerate?
To Reuse or to Incinerate? A case study of the environmental impacts of two alternative waste management strategies for household textile waste in nine municipalities in northern Stockholm, Sweden Robert Bodin, MSc candidate Master of Science Thesis Stockholm 2016 Abstract With an increasing human population in the world, textiles are part of current unsustainable consumption patterns. Unlike most other mass produced products available today however, textiles are often vital to satisfy human core needs, and cannot be considered superfluous. Textile materials can be problematic from an environmental perspective. Synthetics are made from non-renewable petroleum, while production natural textile materials are very resource intensive, and rely on non-renewable energy supplies. Many reports on textiles indicate that production and use have great environmental impacts compared to waste management. On the other hand, it is in the latter phase decided whether the textile should be reused, recycled or discarded. These different material flow alternatives greatly determine overall impacts, since the possibility of avoided production through reuse and recycling is an important factor to consider. The main goal of this report was, through the use of life cycle assessment (LCA), to evaluate the environmental impact of household textile waste management from reuse and disposal alternatives, when conducted through the activities of the Swedish waste management company SÖRAB. Two different waste management strategies/scenarios where compared: one centered around incineration of textile waste, specified as the incineration scenario, and one focused on a textile waste flow where the textiles are separated from household waste and sorted for reuse, recycling and incineration, specified as the reuse scenario. -

A CHALLENGING DIALOGUE Ezequiel
Theme 1 Session 3 HERITAGE ASSESSMENT AND REGIONAL DEVELOPMENT : A CHALLENGING DIALOGUE Ezequiel M. Pinto-Guillaume Archeologist and Illustrator, WSP Sverige [email protected] Abstract. A dialogue with those who intend to develop a site is always challenging. As heritage curators, we must often give advice that developers may not want to hear because it might interfere with the realization of a project. In our work as environmental planners at WSP, we have experienced that it is difficult but of utmost importance to create an open dialogue with those involved in the development of sensitive cultural heritage areas at a regional level. However, we have realized that theory and practice do not always go hand in hand. In this paper, we will take a closer look at two recent projects in which we carried out a cultural and historical assessment and, also, an environmental impact assessment (cultural and historical as well as environmental impact assessments). In both, the team aimed to maintain a dynamic and functional relationship between the existing heritage and the planned future development of the areas. We will see how the outcome of both projects call for a re-examination of the importance of our preliminary assessments. We also believe Insight: how cultural heritage is managed in Sweden In order to understand how regional planning works overall level, the County Administrative Board super- we must first take a look at the organization of heri- vises the issues, and the municipalities deal with the tage protection in Sweden. At a national level it is the issues at a local level. -

Government Communication 2011/12:56 a Coordinated Long-Term Strategy for Roma Skr
Government communication 2011/12:56 A coordinated long-term strategy for Roma Skr. inclusion 2012–2032 2011/12:56 The Government hereby submits this communication to the Riksdag. Stockholm, 16 February 2012 Fredrik Reinfeldt Erik Ullenhag (Ministry of Employment) Key contents of the communication This communication presents a coordinated and long-term strategy for Roma inclusion for the period 2012–2032. The strategy includes investment in development work from 2012–2015, particularly in the areas of education and employment, for which the Government has earmarked funding (Govt. Bill. 2011/12:1, Report 2011/12:KU1, Riksdag Communication 2011/12:62). The twenty-year strategy forms part of the minority policy strategy (prop. 2008/09:158) and is to be regarded as a strengthening of this minority policy (Govt. Bill 1998/99:143). The target group is above all those Roma who are living in social and economic exclusion and are subjected to discrimination. The whole implementation of the strategy should be characterised by Roma participation and Roma influence, focusing on enhancing and continuously monitoring Roma access to human rights at the local, regional and national level. The overall goal of the twenty-year strategy is for a Roma who turns 20 years old in 2032 to have the same opportunities in life as a non-Roma. The rights of Roma who are then twenty should be safeguarded within regular structures and areas of activity to the same extent as are the rights for twenty-year-olds in the rest of the population. This communication broadly follows proposals from the Delegation for Roma Issues in its report ‘Roma rights — a strategy for Roma in Sweden’ (SOU 2010:55), and is therefore also based on various rights laid down in international agreements on human rights, i.e. -

What Protection of the Right to Housing Do Illegal Occupiers Enjoy?
Södertörn University | School of Social Sciences Master Thesis 30 ECTS | Public Law | Autumn Semester 2014 (Master Programme in Human Rights and Public Administration) What Protection of the Right to b Housing do Illegal Occupiers Enjoy? A Study of the Protection in Sweden in Light of the Example of South Africa By: Natasha Ryan Supervisor: Patricia Jonason Abstract With a history of racial discrimination, which inevitably led to illegal occupations of private and public land and subsequent violent evictions, South Africans have enjoyed constitutional protection against illegal and forcible evictions since the mid-1990s. Sweden, a country considerably less accustomed to illegal occupations, does not guarantee the same protection against forcible evictions. However, the recent influx of Roma from Romania and Bulgaria to Sweden without the means to support themselves has resulted in an increase of illegal occupations and led to evictions from publicly owned land. This paper examines: (i) how the case of South Africa relates to the situation in Sweden, (ii) the extent of legal protection offered by Sweden relating to the right to housing and (iii), whether when carrying out such evictions, Sweden respects European legislation and precedent. The results show that South African and European courts have interpreted the right to housing as a right to alternative accommodation for illegal occupants. With no constitutional right to housing in Sweden, legislation only guarantees the right to housing for holders of a permanent right of residence. For EEA citizens, such as Romanians or Bulgarians, the right of residence is conditional. In the absence of a legal obligation to consider the situation of illegal occupiers or to provide alternative accommodation, there seems to be a gap in Swedish legislation; leading to a failure on the behalf of the state to adhere to European commitments and to protect those most vulnerable in society. -

Smart Territorial Mapping for Enhanced Multilevel Governance of S3: Inputs for Inter-Regional Learning Policy Learning Document
Integrating the territorial dimension for cohesive S3 Smart territorial mapping for enhanced multilevel governance of S3: Inputs for inter-regional learning Policy learning document October 2020 Prepared by Ainhoa Arrona, Miren Estensoro, Miren Larrea and James R. Wilson (Orkestra – Basque Institute of Competitiveness) Table of Content 1. Introduction 2 2. Background and aim of the Smart territorial mapping exercise 4 3. Smart territorial mapping: steps 9 3.1. Mapping multilevel specialization and governance 9 3.2. Assessment 10 4. Summary of results of smart territorial mapping exercise in Cohes3ion 12 4.1. The mapping: territorial levels, specialization capabilities, innovation actors and governance spaces 12 4.2. Assessment: strengths and areas of improvement 15 5. Summary 24 Appendixes 25 Appendix 1. Smart territorial map matrix 26 Appendix 2. Smart territorial map Assesment document 27 Appendix 3. Summary of conclusions by partner region 29 Appendix 4. Areas of improvement identified through Smart territorial map exercise in each region grouped by challenge 34 Appendix 5. Smart territorial map: Bizkaia 37 Appendix 6. Smart territorial map: Calabria 47 Appendix 7. Smart territorial map: Mazovia 60 Appendix 8. Smart territorial map: North West Romania 76 Appendix 9. Smart territorial map: Ruhr Metropolis 88 Appendix 10. Smart territorial map: Southern Region Ireland 100 Appendix 11. Smart territorial map: Region Stockholm 119 Appendix 12. Smart territorial map: Wales 133 Smart territorial mapping 1 1. Introduction Regional and national governments play a entrepreneurs) into discovery processes main role as managing authorities of smart supporting more effective policy. While regional specialization strategies (S3) in the European and national governments have a certain Union (EU). -

Adaptation Toolkit for the North Sea Region in a Changing Climate
Adaptation toolkit for the North Sea Region in a changing climate CPA Work Package 4 report August 2011 Table of contents Preface 5 1 Introduction 6 1.1 The project Climate Proof Areas 6 1.2 Aim of this toolkit 7 1.3 Work method and outline of the report 8 1.4 Position of this report in the project 8 Work packages of CPA 9 Connections between the work packages 9 2 Tools for climate proofing 11 2.1 Climate change in the NSR 11 Some of the overall conclusions 12 2.2 Adaptation process 12 2.3 Characteristics of climate proofing 14 Long term decision making and short term measures (time scales) 14 Regional and national scale 14 Uncertainties due to (the sum of) incremental deviations 14 Restricted commitment and low sense of urgency 15 2.4 Problem solving cycle 15 Problem definition 16 Problem specification 16 Generation of solutions 16 Choice 17 Implementation 17 Evaluation 17 2.5 Tools 17 3 Process management 19 3.1 Communications plan 19 Communications plan 19 3.2 Participatory methodology 22 3.3 Process checklists and process guides 23 Matrix based decision support tool 23 4 Problem definition 26 4.1 Description 26 4.2 Overview of tools in the problem definition stage 26 4.3 Recommended tools 31 School projects for creating awareness by future stakeholders 31 2 Keeping climate adaptation on local level: avoiding discussions on a higher abstraction 33 level Regional forum 36 Connecting to local problems and initiatives 40 Defining clear roles and responsibilities in regional alliances 42 4.4 Conclusions 44 5 Problem specification 45 5.1 -

CV Sara Henrysson Eidvall Licensed Psychologist/Specialist Qualification Certificate in IO- Psychology
CV Sara Henrysson Eidvall Licensed psychologist/Specialist Qualification Certificate in IO- psychology Summary Sara Henrysson Eidvall, licensed psychologist, has Swedens Psychologist Association's Specialist Qualification Certificate in Industrial and organizational psychology and is STP certified test user. Sara has worked with development of leaders individually and in groups and management teams in both, governmental, municipal and private organizations. Her clients include municipalities, businesses and government agencies such as the municipality of Vallentuna, Huddinge, Botkyrka, Nacka Municipality, Microsoft, Atrium Ljungberg AB, the Swedish National Board of Institutional Care and Stockholm University. Sara has been responsible for leadership development (leadership programs, management development, team development and individual coaching of managers and experts) in major assignements such as Botkyrka, Huddinge, Nacka, Microsoft and Atrium Ljungberg AB. She is also associate professor in IO-psychology at the departement of psychology at Stockholm university. Education Period Cognitive Behavioral Therapy, CBT, step 1 2005 Specialist Qualification Certificate in IO-psychology 2004 Licensed psychologist 1991 Msc in psychology, Stockholm University 1991 Poppius school of journalism 1992 Social Anthropology, 20 points 1982 Other professional education Quality systems SIS 2014 Viary –smartphone coaching 2013 CBT internet treatment 2013 ACT in theory and practice 2012-2013 ACT at work 2011 ACT and RFT 2010 - 2011 Applied behavior analysis -

Företagsräkningen 1972 / Statistiska Centralbyrån
INLEDNING TILL Företagsräkningen 1972 / Statistiska centralbyrån. – Stockholm : Statistiska centralbyrån, 1975. – (Sveriges officiella statistik). Täckningsår: 1972. Engelsk parallelltitel: The 1972 census of enterprises. Företagsräkningen 1972 består av flera delar, delarnas undertitlar: Del 1. Basdata för företag och myndigheter fördelade efter näringsgren, storlek, samhälssektor, ägarkategori och juridisk form. Part 1. Basic data for enterprises and government departments distributed by major division, institutional sector, type of ownership and legal organization. Del 2 (tre band) Basdata för företag och myndigheters verksamhetsställen fördelade efter näringsgren, storlek, region och ägarkategori. 2:1 Verksamhetsställen totalt och fördelade på riksområden, län och A-regioner. 2:2 Verksamhetsställen fördelade på kommuner; A–M-län 2:3 Verksamhetsställen fördelade på kommuner; N–BD-län Part 2. Basic data for local units of enterprises and government agencises, disstributed by industry (SNI, 1, 2, 3-digit level), size, region and type of ownership. Del 3 Sysselsättnings-, resultat- och kapitaldata för företag inom den affärsdrivande sektorn fördelade efter näringsgren, storlek, ägarkategori och juridisk form. Part 3. Data on employees, profits and capital for enterprises in the business sector distribute by industri, size, type of ownership and legal organization. Del 4 Sysselsättnings- och omsättningsdata för verksamhetsställen inom den affärsdrivande sektorn fördelade eftter näringsgren, storlek och region. Part 4. Data of employees and turnover for local units of enterprises in the business sector distributed by industry, size and region. Appendix Lagstiftning, Klassificeringsstandard, Insamlade data, Blankettförteckning, Blankettexempel. Appendix Föregångare: 1951 års företagsräkning / Kommerskollegium. – Stockholm : Statistiska centralbyrån, 1955. – (Sveriges officiella statistik). Täckningsår: 1951. Engelsk parallelltitel: The 1951 census of production, distribution and services. 1931 års företagsräkning / verkställd av Kommerskollegium, Stockholm 1935. -

Springs in the Central Parts of Vallentuna Municipality Traditions and Threats
DEGREE PROJECT IN CHEMICAL ENGINEERING AND TECHNOLOGY, FIRST LEVEL STOCKHOLM, SWEDEN 2017 Springs in the central parts of Vallentuna municipality Traditions and threats Hannan Hadodo A stream emerging from a spring horizon in Arkels tingstad, Vallentuna KTH ROYAL INSTITUTE OF TECHNOLOGY KTH CHEMICAL SCIENCE AND ENGINEERING Degree Project Bachelor of Science in Chemical Engineering and Technology Title: Springs in the central parts of Vallentuna municipality traditions and threats Swedish title: Källor i centrala Vallentuna traditioner och hot Keywords: Spring, water quality, restoration Workplace: KTH and Vallentuna References in Vallentuna: Anton Mankesjö, Vallentuna kommun Staffan Rosander, Vallentuna Hembygdsförening Anders Eriksson, Källakademin Nicole Sundin, Vallentuna kommun Supervisor: Olle Wahlberg Student: Hannan Hadodo Date: 2017-11-07 Examiner: Lars Kloo 2 Abstract Twenty springs in the central parts of Vallentuna municipality have been studied to determine their present water qualities and suggest actions that could be taken to improve the water quality and availability of the springs. The measured parameters are the water volume, the water flow, the water temperature, the pH-value, the electrical conductivity, the chloride concentration, the alkalinity and the CODMn. The sensory properties of the springs were also examined, which include odour, colour, clarity and precipitation. Measurements of the water flows, the water temperatures, the pH-values and the electrical conductivities were performed during a field trip in Vallentuna. During this field trip, two water samples were taken from each spring. Thereafter, the alkalinity, the chloride concentration, the CODMn and the sensory properties were determined in the laboratory. Four of the springs, which were wells, have water of high quality and can easily be restored.