Accounting for Intraspecific Variation Transforms Our Understanding of Artiodactyl Social Evolution
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inclusion of Asiatic Mammal Species on Cms Appendices
Convention on the Conservation of Migratory Species of Wild Animals Secretariat provided by the United Nations Environment Programme 14 th MEETING OF THE CMS SCIENTIFIC COUNCIL Bonn, Germany, 14-17 March 2007 CMS/ScC14/Doc.13 Agenda item 6(a) DRAFT PROPOSALS FOR THE INCLUSION OF ASIATIC MAMMAL SPECIES ON CMS APPENDICES (Prepared by the Secretariat) 1. The four draft proposals for the amendment of CMS Appendices attached to this note have been prepared by the Institut Royal des Sciences Naturelles de Belgique and have been submitted by Dr. Pierre Devillers, Scientific Councillor for the European Community and vice-chairman of the Scientific Council. 2. Preparation of these draft proposals is undertaken within the Central Eurasian Aridland Concerted Action and associated Cooperative Action approved by the 8 th Meeting of the Conference of the Parties to CMS (Recommendation 8.23), covering threatened migratory large mammals of the temperate and cold deserts, semi-deserts, steppes and associated mountains of Central Asia, the Northern Indian sub-continent, Western Asia, the Caucasus and Eastern Europe. 3. In particular, Rec. 8.23 “encourages Range States and other interested Parties to prepare, in cooperation with the Scientific Council and the Secretariat, the necessary proposals to include in Appendix I or Appendix II threatened species that would benefit from the Action”. For reasons of economy, documents are printed in a limited number, and will not be distributed at the meeting. Delegates are kindly requested to bring their copy to the meeting and not to request additional copies. DRAFT PROPOSAL FOR INCLUSION OF SPECIES ON THE APPENDICES OF THE CONVENTION ON THE CONSERVATION OF MIGRATORY SPECIES OF WILD ANIMALS Proposal to add in Appendix I Pantholops hodgsonii Document largely based on the species information provided in IUCN Redlist of Threatened Species database (2006) February 2007 2 1. -

Pending World Record Waterbuck Wins Top Honor SC Life Member Susan Stout Has in THIS ISSUE Dbeen Awarded the President’S Cup Letter from the President
DSC NEWSLETTER VOLUME 32,Camp ISSUE 5 TalkJUNE 2019 Pending World Record Waterbuck Wins Top Honor SC Life Member Susan Stout has IN THIS ISSUE Dbeen awarded the President’s Cup Letter from the President .....................1 for her pending world record East African DSC Foundation .....................................2 Defassa Waterbuck. Awards Night Results ...........................4 DSC’s April Monthly Meeting brings Industry News ........................................8 members together to celebrate the annual Chapter News .........................................9 Trophy and Photo Award presentation. Capstick Award ....................................10 This year, there were over 150 entries for Dove Hunt ..............................................12 the Trophy Awards, spanning 22 countries Obituary ..................................................14 and almost 100 different species. Membership Drive ...............................14 As photos of all the entries played Kid Fish ....................................................16 during cocktail hour, the room was Wine Pairing Dinner ............................16 abuzz with stories of all the incredible Traveler’s Advisory ..............................17 adventures experienced – ibex in Spain, Hotel Block for Heritage ....................19 scenic helicopter rides over the Northwest Big Bore Shoot .....................................20 Territories, puku in Zambia. CIC International Conference ..........22 In determining the winners, the judges DSC Publications Update -

Population, Distribution and Conservation Status of Sitatunga (Tragelaphus Spekei) (Sclater) in Selected Wetlands in Uganda
POPULATION, DISTRIBUTION AND CONSERVATION STATUS OF SITATUNGA (TRAGELAPHUS SPEKEI) (SCLATER) IN SELECTED WETLANDS IN UGANDA Biological -Life history Biological -Ecologicl… Protection -Regulation of… 5 Biological -Dispersal Protection -Effectiveness… 4 Biological -Human tolerance Protection -proportion… 3 Status -National Distribtuion Incentive - habitat… 2 Status -National Abundance Incentive - species… 1 Status -National… Incentive - Effect of harvest 0 Status -National… Monitoring - confidence in… Status -National Major… Monitoring - methods used… Harvest Management -… Control -Confidence in… Harvest Management -… Control - Open access… Harvest Management -… Control of Harvest-in… Harvest Management -Aim… Control of Harvest-in… Harvest Management -… Control of Harvest-in… Tragelaphus spekii (sitatunga) NonSubmitted Detrimental to Findings (NDF) Research and Monitoring Unit Uganda Wildlife Authority (UWA) Plot 7 Kira Road Kamwokya, P.O. Box 3530 Kampala Uganda Email/Web - [email protected]/ www.ugandawildlife.org Prepared By Dr. Edward Andama (PhD) Lead consultant Busitema University, P. O. Box 236, Tororo Uganda Telephone: 0772464279 or 0704281806 E-mail: [email protected] [email protected], [email protected] Final Report i January 2019 Contents ACRONYMS, ABBREVIATIONS, AND GLOSSARY .......................................................... vii EXECUTIVE SUMMARY ....................................................................................................... viii 1.1Background ........................................................................................................................... -

Inner New Last Final.Pmd
Conservation Status and Population Density of Himalayan Serow (Capricornis sumatraensis) in Annapurna Conservation Area of Nepal Achyut Aryal1 Abstract Himalayan Serow ‘Capricornis sumatraensis’ population is isolated in a small patch of the southern part of Annapurna Conservation Area (ACA) with a population density of 1.17 individual/km2 and a population sex ratio of 1:1.6(Male: Female). A strong correlation was found between population (y) and pellets density (x) (y=0.011x-0.2619, R2-0.97). The major problems in the Serow habitat were habitat fragmentation & land use change, conflict between predator and villager, livestock grazing and poaching. The study was successful in providing information on the present status of Himalayan Serow in the ACA. cGgk'0f{ ;+/If0f If]qsf] blIf0fL efudf ;fgf] ´'08df ! .!& k|lt ju { ls=ld= hg3gTj / ! :! .^ (k'lnË::qLlnË) sf] cgkftdf' 5l§Psf' ] ;fgf ] o;sf ] hg;Vof+ PSnf?kdf] /xsf] ] 5 . hg;ªVof\ / jsfnfsf{} ] 3gTjlar 3lx/f ] ;DaGw /xsf] ] kfOof] . lxdfnog l;/f]sf] ;/+If0fsf d'Vo ;d:ofx?df jf;:yfg 6'lqmg' / hldgsf] k|of]udf abnfj, ufpFn] ljrsf] åGå, ufO{j:t' rl/r/0f / u}/sfg'gL lzsf/ x'g\ . lxdfnog l;/f] ;+/If0fsf nflu ;+/If0f lzIff, hgtfdf´ o;af/] r]tgf hufpg] sfo{qmd cfjZos b]lvPsf 5g\ . of] cWoogn] cGgk'0f{ If]qdf /xsf] lxdfnog l;/f]sf] cj:yfjf/] hfgsf/L lbg ;kmn ePsf] 5 . Key Words: Population density, Sex ratio, Fragmentation, Conservation, Threats Introduction Himalayan Serow 'Capricornis sumatraensis' (hereafter Serow) is a threatened animal, listed in Appendix I by CITES and class as "Vulnerable" by IUCN Red data (IUCN, 2004). -

08E – CHAMOIS (GAMSWILD)
U.S. Forces Europe Hunting, Fishing, and Sport Shooting Program Guide to Hunting in Germany 08e – CHAMOIS (GAMSWILD) Breeding season (Brunftzeit): November Gestation period: 20-21 weeks Young are born: May (one, sometimes two) Teeth: 32 Males: Gamsbock Females: Geiss Fawns: Kitz Herd: Rudel Based on fossils, scientists believe that some 100,000 years ago, ancestors of chamois known in Europe today originally migrated from Central Asia. These populations were wiped out during the last ice-age, except for chamois that are located in the Apennine and the Pyrenees mountain ranges. When the glaciers melted away, about 10,000 years ago, another Chamois migration from Asia led to a re-population of Eastern and Central Europe, except for Scandinavia and the British Islands. 08e-1 U.S. Forces Europe Hunting, Fishing, and Sport Shooting Program Guide to Hunting in Germany As a true mountain animal, Chamois lived mainly in regions with rocks and steep cliffs and outside the forest. With the increasing extension of forest on the former taiga landscape, Chamois became restricted to Alpine areas. In this sense, “Alpine” means regions above the forest line. These were mainly the Carpathians, the Tatras, the Dinarids, the Alps, the Apennines, and the Pyrenees. Aside from alpine regions, Chamois have also been introduced successfully to some parts in Europe (Schwarzwald and Elbsandsteingebirge, Germany), and to New Zealand. The chamois is a member of the family Bovidae. Chamois is a very distinct genus, with no close relatives in the world of cloven hoofed game. The Serau and the Goral, both in Central Asia, and the Snow Goat in North America are considered the closest relatives. -

The Eastern Cape –
The Eastern Cape – Revisited By Jeff Belongia A return to South Africa’s Eastern Cape was inevitable – the idea already firmly planted in my mind since my first visit in 1985. Africa, in general, has a wonderful yet strange control over the soul, and many writers have tried to express the reasoning behind it. I note this captivation and recognize the allure, and am too weak to resist. For me, Africa is what dreams are made of – and I dream of it daily. Dr. Martin Luther King was wise when he chose the phrase, “I have a dream.” He could have said, “I have a strategic plan.” Not quite the same effect! People follow their dreams. As parents we should spend more time teaching our children to dream, and to dream big. There is no Standard Operating Procedure to get through the tough times in life. Strength and discipline are measured by the depth and breadth of our dreams and not by strategic planning! The Catholic nuns at Saint Peter’s grade school first noted my talents. And they all told me to stop daydreaming. But I’ve never been able to totally conquer that urge – I dream continually of the romance of Africa, including the Eastern Cape. The Eastern Cape is a special place with a wide variety of antelope – especially those ‘pygmy’ species found nowhere else on the continent: Cape grysbok, blue duiker, Vaal rhebok, steenbok, grey duiker, suni, and oribi. Still not impressed? Add Cape bushbuck, mountain reedbuck, blesbok, nyala, bontebok, three colour phases of the Cape springbok, and great hunting for small cats such as caracal and serval. -

Capture, Restraint and Transport Stress in Southern Chamois (Rupicapra Pyrenaica)
Capture,Capture, restraintrestraint andand transporttransport stressstress ininin SouthernSouthern chamoischamois ((RupicapraRupicapra pyrenaicapyrenaica)) ModulationModulation withwith acepromazineacepromazine andand evaluationevaluation usingusingusing physiologicalphysiologicalphysiological parametersparametersparameters JorgeJorgeJorge RamónRamónRamón LópezLópezLópez OlveraOlveraOlvera 200420042004 Capture, restraint and transport stress in Southern chamois (Rupicapra pyrenaica) Modulation with acepromazine and evaluation using physiological parameters Jorge Ramón López Olvera Bellaterra 2004 Esta tesis doctoral fue realizada gracias a la financiación de la Comisión Interministerial de Ciencia y Tecnología (proyecto CICYT AGF99- 0763-C02) y a una beca predoctoral de Formación de Investigadores de la Universidad Autónoma de Barcelona, y contó con el apoyo del Departament de Medi Ambient de la Generalitat de Catalunya. Los Doctores SANTIAGO LAVÍN GONZÁLEZ e IGNASI MARCO SÁNCHEZ, Catedrático de Universidad y Profesor Titular del Área de Conocimiento de Medicina y Cirugía Animal de la Facultad de Veterinaria de la Universidad Autónoma de Barcelona, respectivamente, CERTIFICAN: Que la memoria titulada ‘Capture, restraint and transport stress in Southern chamois (Rupicapra pyrenaica). Modulation with acepromazine and evaluation using physiological parameters’, presentada por el licenciado Don JORGE R. LÓPEZ OLVERA para la obtención del grado de Doctor en Veterinaria, se ha realizado bajo nuestra dirección y, considerándola satisfactoriamente -

War and the White Rhinos
War and the White Rhinos Kai Curry-Lindahl Until 1963 the main population of the northern square-lipped (white) rhino was in the Garamba National Park, in the Congo (now Zaire) where they had increased to over 1200. That year armed rebels occupied the park, and when three years later they had been driven out, the rhinos had been drastically reduced: numbers were thought to be below 50. Dr. Curry-Iindahl describes what he found in 1966 and 1967. The northern race of the square-lipped rhinoceros Ceratotherium simum cottoni was once widely distributed in Africa north of the equator, but persecution has exterminated it over large areas. It is now known to occur only in south-western Sudan, north-eastern Congo (Kinshasa) and north-western Uganda. It is uncertain whether it still exists in northern Ubangui, in the Central African Republic. In the Sudan, where for more than ten years its range has been affected by war and serious disturbances, virtually nothing is known of its present status. In Uganda numbers dropped from about 350 in 1955 to 80 in 1962 and about 20-25 in 1969 (Cave 1963, Simon 1970); the twelve introduced into the Murchison Falls National Park in 1960, despite two being poached, increased to 18 in 1971. But the bulk of the population before 1963 was in the Garamba National Park in north-eastern Congo, in the Uele area. There, since 1938, it had been virtually undisturbed, and, thanks to the continuous research which characterised the Congo national parks before 1960, population figures are known for several periods (see the Table on page 264). -

Serologic Surveys for Natural Foci of Contagious Ecthyma Infection
FI.NAL REP.ORT (R.ESEARCH) State: Alaska Cooperators: Randall L. Zarnke and Kenneth A. Neiland Project No.: W-21-1 Project Title: Big Game Investigations Job No.: l8.1R Job Title: Serologic Surveys for Natural Foci of contag1ous Ecthyma Infection Period Covered: July 1, 1979 to J~~? ~0, 1~80 SUMMARY Contagious ecthyma (C. E. ) antibody prevalence in domestic sheep and goats in Interior Alaska du~ing 1978 was quite low (7-10%). This suggested a low level of transmission of the virus among these species during this time period. C. E. antibody prevalence in Dall sheep increased .from 30 percent in 1971 to 100 percent in 1978. This suggested an increased level of transmission to, and/or among, these animals during this period. Following a C.E. epizootic in a band of captive Dall sheep in 1977, antibody prevalence was 80 percent in this group of sheep. Less than l year later, prevalence had dropped to 10 percent in the ·same band. Thus it appeared that antibody produced in response to this . strain of C.E. is short-lived. Antibody was also detected in 10 of 22 free ranging muskoxen taken by sport hunters on Nunivak Island in 1978. Detectable antibody levels were not found in any of 19 muskoxen captured on the island in 1979. No antibody was found in a small number of captive muskoxen from Unalakleet. Several of the captive muskoxen were ·known to have been infected with C.E. within 1 to 2 years prior to the time of sampling. This further substantiates the belief that anti body produced in response to infection by the Alaskan strain of C.E. -
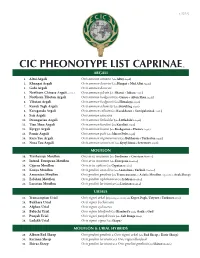
Cic Pheonotype List Caprinae©
v. 5.25.12 CIC PHEONOTYPE LIST CAPRINAE © ARGALI 1. Altai Argali Ovis ammon ammon (aka Altay Argali) 2. Khangai Argali Ovis ammon darwini (aka Hangai & Mid Altai Argali) 3. Gobi Argali Ovis ammon darwini 4. Northern Chinese Argali - extinct Ovis ammon jubata (aka Shansi & Jubata Argali) 5. Northern Tibetan Argali Ovis ammon hodgsonii (aka Gansu & Altun Shan Argali) 6. Tibetan Argali Ovis ammon hodgsonii (aka Himalaya Argali) 7. Kuruk Tagh Argali Ovis ammon adametzi (aka Kuruktag Argali) 8. Karaganda Argali Ovis ammon collium (aka Kazakhstan & Semipalatinsk Argali) 9. Sair Argali Ovis ammon sairensis 10. Dzungarian Argali Ovis ammon littledalei (aka Littledale’s Argali) 11. Tian Shan Argali Ovis ammon karelini (aka Karelini Argali) 12. Kyrgyz Argali Ovis ammon humei (aka Kashgarian & Hume’s Argali) 13. Pamir Argali Ovis ammon polii (aka Marco Polo Argali) 14. Kara Tau Argali Ovis ammon nigrimontana (aka Bukharan & Turkestan Argali) 15. Nura Tau Argali Ovis ammon severtzovi (aka Kyzyl Kum & Severtzov Argali) MOUFLON 16. Tyrrhenian Mouflon Ovis aries musimon (aka Sardinian & Corsican Mouflon) 17. Introd. European Mouflon Ovis aries musimon (aka European Mouflon) 18. Cyprus Mouflon Ovis aries ophion (aka Cyprian Mouflon) 19. Konya Mouflon Ovis gmelini anatolica (aka Anatolian & Turkish Mouflon) 20. Armenian Mouflon Ovis gmelini gmelinii (aka Transcaucasus or Asiatic Mouflon, regionally as Arak Sheep) 21. Esfahan Mouflon Ovis gmelini isphahanica (aka Isfahan Mouflon) 22. Larestan Mouflon Ovis gmelini laristanica (aka Laristan Mouflon) URIALS 23. Transcaspian Urial Ovis vignei arkal (Depending on locality aka Kopet Dagh, Ustyurt & Turkmen Urial) 24. Bukhara Urial Ovis vignei bocharensis 25. Afghan Urial Ovis vignei cycloceros 26. -

Bulletin of the United States Fish Commission Seattlenwf
THE FUR SEALS AND OTHER LIFE OF THE PRIBILOF ISLANDS, ALASKA, IN 1914 By Wilfred H. Osgood, Edward A. Preble, and George H. Parker I Blank page retained for pagination CONTENTS. Palll!. LgTTERS OF TRANSMITTAL........•...........'............................................. II LETTER OF SUBMITTAL................... ................................................. 12 INTRODUCTION............... .. 13 Personnel and instructions. ............................................................. 13 Investigations by Canada and Japan... .. .. .. .. .. .. .. IS Itinerary , .. " . .. IS Impartial nature of the investigation " '" . 16 Acknowledgments................. 16 THg PRIBILOF ISLANDS..................................................................... 17 General description...................... .. 17 Vegetation. 18 Climate............. 18 CHARACTER AND HABITS OF THE FUR SEAL IN BRIEF....... ................................. 18 G'eneral characteristics. ................ .. .............................................. 18 Range.................................................................................. 18 , Breeding habits ; ........................ 19 Habits of bachelors : ......................... 20 Age of seals... ;. ....................................................................... 20 SEALING HISTORY IN BRIEF................................................................. 21 Russian management " .. .. 21 American occupation and the leasing system : . .. ... 21 The growth of pelagic sealing , "".. .. .. .. 22 The Paris Tribunal -

Reproductive Seasonality in Captive Wild Ruminants: Implications for Biogeographical Adaptation, Photoperiodic Control, and Life History
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2012 Reproductive seasonality in captive wild ruminants: implications for biogeographical adaptation, photoperiodic control, and life history Zerbe, Philipp Abstract: Zur quantitativen Beschreibung der Reproduktionsmuster wurden Daten von 110 Wildwiederkäuer- arten aus Zoos der gemässigten Zone verwendet (dabei wurde die Anzahl Tage, an denen 80% aller Geburten stattfanden, als Geburtenpeak-Breite [BPB] definiert). Diese Muster wurden mit verschiede- nen biologischen Charakteristika verknüpft und mit denen von freilebenden Tieren verglichen. Der Bre- itengrad des natürlichen Verbreitungsgebietes korreliert stark mit dem in Menschenobhut beobachteten BPB. Nur 11% der Spezies wechselten ihr reproduktives Muster zwischen Wildnis und Gefangenschaft, wobei für saisonale Spezies die errechnete Tageslichtlänge zum Zeitpunkt der Konzeption für freilebende und in Menschenobhut gehaltene Populationen gleich war. Reproduktive Saisonalität erklärt zusätzliche Varianzen im Verhältnis von Körpergewicht und Tragzeit, wobei saisonalere Spezies für ihr Körpergewicht eine kürzere Tragzeit aufweisen. Rückschliessend ist festzuhalten, dass Photoperiodik, speziell die abso- lute Tageslichtlänge, genetisch fixierter Auslöser für die Fortpflanzung ist, und dass die Plastizität der Tragzeit unterstützend auf die erfolgreiche Verbreitung der Wiederkäuer in höheren Breitengraden wirkte. A dataset on 110 wild ruminant species kept in captivity in temperate-zone zoos was used to describe their reproductive patterns quantitatively (determining the birth peak breadth BPB as the number of days in which 80% of all births occur); then this pattern was linked to various biological characteristics, and compared with free-ranging animals. Globally, latitude of natural origin highly correlates with BPB observed in captivity, with species being more seasonal originating from higher latitudes.