Reproductive Seasonality in Captive Wild Ruminants: Implications for Biogeographical Adaptation, Photoperiodic Control, and Life History
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Habitat Preference of Himalayan Musk Deer (Moschus Leucogaster Hodgson, 1839) at Lapchi of Bigu Rural Municipality, Gaurishankar Conservation Area
21 Nep J Environ Sci (2021), 9(1), 21-28 ISSN 2350-8647 (Print) 2542-2901 (Online) https://doi.org/10.3126/njes.v9i1.37844 Research Article Habitat preference of Himalayan musk deer (Moschus leucogaster Hodgson, 1839) at Lapchi of Bigu Rural Municipality, Gaurishankar Conservation Area Narayan Prasad Koju1,2,*, Bijay Bashyal3, Satya Narayan Shah1,4 1 Center for Post Graduate Studies, Nepal Engineering College, Pokhara University, Nepal 2 Department of Psychology, University of Washington, Seattle, USA 3 Central Department of Environmental Science, Tribhuvan University, Kathmandu 4 Gaurishankar Conservation Area Project, National Trust of Nature Conservation (Received: 06 June 2021; Revised: 02 July 2021; Accepted: 03 July 2021) Abstract The Himalayan musk deer (Moschus leucogaster) is an endangered species listed in the IUCN Red List and Appendix I of CITES. It is widely but discontinuously distributed in Nepal. A Pellet sign survey was carried in April 2019 in Lapchi valley of Gaurishankar Conservation Area (GCA) in Nepal to assess the habitat preference of Himalayan musk deer. A total of 11 transects of 16348 m length and 10 m wide was surveyed. Seven Parameters: Elevation, Aspect, ground cover, distance from the water source, crown cover, rock exposure, and distance from settlement/cow sheds were recorded from the location where pellet (toilet) of musk deer were recorded to extrapolate the probable habitat map. We recorded a total of 157 musk deer pellet groups in the study area14.27 ± 2.91. The study concluded that the 38.4% (26.5 km2) area of Lapchi valley is the probable habitat of musk deer. The 2 – test suggested that the distribution of musk deer is significantly associated with elevation and aspect of the location. -

Population, Distribution and Conservation Status of Sitatunga (Tragelaphus Spekei) (Sclater) in Selected Wetlands in Uganda
POPULATION, DISTRIBUTION AND CONSERVATION STATUS OF SITATUNGA (TRAGELAPHUS SPEKEI) (SCLATER) IN SELECTED WETLANDS IN UGANDA Biological -Life history Biological -Ecologicl… Protection -Regulation of… 5 Biological -Dispersal Protection -Effectiveness… 4 Biological -Human tolerance Protection -proportion… 3 Status -National Distribtuion Incentive - habitat… 2 Status -National Abundance Incentive - species… 1 Status -National… Incentive - Effect of harvest 0 Status -National… Monitoring - confidence in… Status -National Major… Monitoring - methods used… Harvest Management -… Control -Confidence in… Harvest Management -… Control - Open access… Harvest Management -… Control of Harvest-in… Harvest Management -Aim… Control of Harvest-in… Harvest Management -… Control of Harvest-in… Tragelaphus spekii (sitatunga) NonSubmitted Detrimental to Findings (NDF) Research and Monitoring Unit Uganda Wildlife Authority (UWA) Plot 7 Kira Road Kamwokya, P.O. Box 3530 Kampala Uganda Email/Web - [email protected]/ www.ugandawildlife.org Prepared By Dr. Edward Andama (PhD) Lead consultant Busitema University, P. O. Box 236, Tororo Uganda Telephone: 0772464279 or 0704281806 E-mail: [email protected] [email protected], [email protected] Final Report i January 2019 Contents ACRONYMS, ABBREVIATIONS, AND GLOSSARY .......................................................... vii EXECUTIVE SUMMARY ....................................................................................................... viii 1.1Background ........................................................................................................................... -

Number of Living Species in Australia and the World
Numbers of Living Species in Australia and the World 2nd edition Arthur D. Chapman Australian Biodiversity Information Services australia’s nature Toowoomba, Australia there is more still to be discovered… Report for the Australian Biological Resources Study Canberra, Australia September 2009 CONTENTS Foreword 1 Insecta (insects) 23 Plants 43 Viruses 59 Arachnida Magnoliophyta (flowering plants) 43 Protoctista (mainly Introduction 2 (spiders, scorpions, etc) 26 Gymnosperms (Coniferophyta, Protozoa—others included Executive Summary 6 Pycnogonida (sea spiders) 28 Cycadophyta, Gnetophyta under fungi, algae, Myriapoda and Ginkgophyta) 45 Chromista, etc) 60 Detailed discussion by Group 12 (millipedes, centipedes) 29 Ferns and Allies 46 Chordates 13 Acknowledgements 63 Crustacea (crabs, lobsters, etc) 31 Bryophyta Mammalia (mammals) 13 Onychophora (velvet worms) 32 (mosses, liverworts, hornworts) 47 References 66 Aves (birds) 14 Hexapoda (proturans, springtails) 33 Plant Algae (including green Reptilia (reptiles) 15 Mollusca (molluscs, shellfish) 34 algae, red algae, glaucophytes) 49 Amphibia (frogs, etc) 16 Annelida (segmented worms) 35 Fungi 51 Pisces (fishes including Nematoda Fungi (excluding taxa Chondrichthyes and (nematodes, roundworms) 36 treated under Chromista Osteichthyes) 17 and Protoctista) 51 Acanthocephala Agnatha (hagfish, (thorny-headed worms) 37 Lichen-forming fungi 53 lampreys, slime eels) 18 Platyhelminthes (flat worms) 38 Others 54 Cephalochordata (lancelets) 19 Cnidaria (jellyfish, Prokaryota (Bacteria Tunicata or Urochordata sea anenomes, corals) 39 [Monera] of previous report) 54 (sea squirts, doliolids, salps) 20 Porifera (sponges) 40 Cyanophyta (Cyanobacteria) 55 Invertebrates 21 Other Invertebrates 41 Chromista (including some Hemichordata (hemichordates) 21 species previously included Echinodermata (starfish, under either algae or fungi) 56 sea cucumbers, etc) 22 FOREWORD In Australia and around the world, biodiversity is under huge Harnessing core science and knowledge bases, like and growing pressure. -

Wild About Learning
WILD ABOUT LEARNING An Interdisciplinary Unit Fostering Discovery Learning Written on a 4th grade reading level, Wild Discoveries: Wacky New Animals, is perfect for every kid who loves wacky animals! With engaging full-color photos throughout, the book draws readers right into the animal action! Wild Discoveries features newly discovered species from around the world--such as the Shocking Pink Dragon and the Green Bomber. These wacky species are organized by region with fun facts about each one's amazing abilities and traits. The book concludes with a special section featuring new species discovered by kids! Heather L. Montgomery writes about science and nature for kids. Her subject matter ranges from snake tongues to snail poop. Heather is an award-winning teacher who uses yuck appeal to engage young minds. During a typical school visit, petrified parts and tree guts inspire reluctant writers and encourage scientific thinking. Heather has a B.S. in Biology and a M.S. in Environmental Education. When she is not writing, you can find her painting her face with mud at the McDowell Environmental Center where she is the Education Coordinator. Heather resides on the Tennessee/Alabama border. Learn more about her ten books at www.HeatherLMontgomery.com. Dear Teachers, Photo by Sonya Sones As I wrote Wild Discoveries: Wacky New Animals, I was astounded by how much I learned. As expected, I learned amazing facts about animals and the process of scientifically describing new species, but my knowledge also grew in subjects such as geography, math and language arts. I have developed this unit to share that learning growth with children. -

Review of Asian Species/Country Combinations Subject to Long-Standing Import Suspensions
Review of Asian species/country combinations subject to long-standing import suspensions (Version edited for public release) SRG 54 Prepared for the European Commission Directorate General Environment ENV.E.2. – Environmental Agreements and Trade by the United Nations Environment Programme World Conservation Monitoring Centre November, 2010 UNEP World Conservation Monitoring PREPARED FOR Centre 219 Huntingdon Road The European Commission, Brussels, Belgium Cambridge CB3 0DL DISCLAIMER United Kingdom Tel: +44 (0) 1223 277314 The contents of this report do not necessarily Fax: +44 (0) 1223 277136 reflect the views or policies of UNEP or Email: [email protected] Website: www.unep-wcmc.org contributory organisations. The designations employed and the presentations do not imply ABOUT UNEP-WORLD CONSERVATION the expressions of any opinion whatsoever on MONITORING CENTRE the part of UNEP, the European Commission or contributory organisations concerning the The UNEP World Conservation Monitoring legal status of any country, territory, city or Centre (UNEP-WCMC), based in Cambridge, area or its authority, or concerning the UK, is the specialist biodiversity information delimitation of its frontiers or boundaries. and assessment centre of the United Nations Environment Programme (UNEP), run cooperatively with WCMC, a UK charity. The © Copyright: 2010, European Commission Centre's mission is to evaluate and highlight the many values of biodiversity and put authoritative biodiversity knowledge at the centre of decision-making. Through the analysis and synthesis of global biodiversity knowledge the Centre provides authoritative, strategic and timely information for conventions, countries and organisations to use in the development and implementation of their policies and decisions. The UNEP-WCMC provides objective and scientifically rigorous procedures and services. -

Gaits and Their Development in the Infraorder Pecora by Anne Innis
Gaits and Their Development in the Infraorder Pecora by Anne Innis Dagg, M.A. THESIS Presented to the Faculty of Graduate Studies of the University of Waterloo in partial ful fillmenc of the requirements for the degree of Doctor of Philosopty in Biolog The University of Waterloo February, 1967 The University of Waterloo requires the signature of all persons using this thesis. Please sign below, and give address and date. I hereby declare that I am the sole a�thor of this thesis. I authorize the University of Waterloo to lend it to other institutions or individuals for the purpose of scholarly research. Signature Abstract The gaits of twenty-eight species of the Infraorder Pecora are analyzed from motion picture sequences comprising over 45,000 frames. For each gait the percentage time spent on the various supporting legs during each stride and the order in which the combinations of supporting legs were used are tabulated. When possible the times for the strides are calculated. The walk patterns of each of the four families studied are shown to be statistically distinctive. Within each family, those members that live where the vegetation is so dense that they must rely on hearing to warn them of danger use a more stable walk than those species that inhabit open grasslands. A stable walk is that in which diagonal legs are used in·preference to lateral legs and three or four supporting legs are used rather than two. With a more stable walk, 4 species is able to pause quickly and to flee instantly if danger thr�atens. -

Birds of the East Texas Baptist University Campus with Birds Observed Off-Campus During BIOL3400 Field Course
Birds of the East Texas Baptist University Campus with birds observed off-campus during BIOL3400 Field course Photo Credit: Talton Cooper Species Descriptions and Photos by students of BIOL3400 Edited by Troy A. Ladine Photo Credit: Kenneth Anding Links to Tables, Figures, and Species accounts for birds observed during May-term course or winter bird counts. Figure 1. Location of Environmental Studies Area Table. 1. Number of species and number of days observing birds during the field course from 2005 to 2016 and annual statistics. Table 2. Compilation of species observed during May 2005 - 2016 on campus and off-campus. Table 3. Number of days, by year, species have been observed on the campus of ETBU. Table 4. Number of days, by year, species have been observed during the off-campus trips. Table 5. Number of days, by year, species have been observed during a winter count of birds on the Environmental Studies Area of ETBU. Table 6. Species observed from 1 September to 1 October 2009 on the Environmental Studies Area of ETBU. Alphabetical Listing of Birds with authors of accounts and photographers . A Acadian Flycatcher B Anhinga B Belted Kingfisher Alder Flycatcher Bald Eagle Travis W. Sammons American Bittern Shane Kelehan Bewick's Wren Lynlea Hansen Rusty Collier Black Phoebe American Coot Leslie Fletcher Black-throated Blue Warbler Jordan Bartlett Jovana Nieto Jacob Stone American Crow Baltimore Oriole Black Vulture Zane Gruznina Pete Fitzsimmons Jeremy Alexander Darius Roberts George Plumlee Blair Brown Rachel Hastie Janae Wineland Brent Lewis American Goldfinch Barn Swallow Keely Schlabs Kathleen Santanello Katy Gifford Black-and-white Warbler Matthew Armendarez Jordan Brewer Sheridan A. -
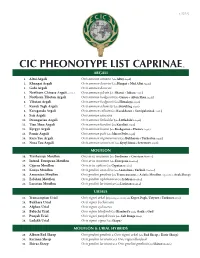
Cic Pheonotype List Caprinae©
v. 5.25.12 CIC PHEONOTYPE LIST CAPRINAE © ARGALI 1. Altai Argali Ovis ammon ammon (aka Altay Argali) 2. Khangai Argali Ovis ammon darwini (aka Hangai & Mid Altai Argali) 3. Gobi Argali Ovis ammon darwini 4. Northern Chinese Argali - extinct Ovis ammon jubata (aka Shansi & Jubata Argali) 5. Northern Tibetan Argali Ovis ammon hodgsonii (aka Gansu & Altun Shan Argali) 6. Tibetan Argali Ovis ammon hodgsonii (aka Himalaya Argali) 7. Kuruk Tagh Argali Ovis ammon adametzi (aka Kuruktag Argali) 8. Karaganda Argali Ovis ammon collium (aka Kazakhstan & Semipalatinsk Argali) 9. Sair Argali Ovis ammon sairensis 10. Dzungarian Argali Ovis ammon littledalei (aka Littledale’s Argali) 11. Tian Shan Argali Ovis ammon karelini (aka Karelini Argali) 12. Kyrgyz Argali Ovis ammon humei (aka Kashgarian & Hume’s Argali) 13. Pamir Argali Ovis ammon polii (aka Marco Polo Argali) 14. Kara Tau Argali Ovis ammon nigrimontana (aka Bukharan & Turkestan Argali) 15. Nura Tau Argali Ovis ammon severtzovi (aka Kyzyl Kum & Severtzov Argali) MOUFLON 16. Tyrrhenian Mouflon Ovis aries musimon (aka Sardinian & Corsican Mouflon) 17. Introd. European Mouflon Ovis aries musimon (aka European Mouflon) 18. Cyprus Mouflon Ovis aries ophion (aka Cyprian Mouflon) 19. Konya Mouflon Ovis gmelini anatolica (aka Anatolian & Turkish Mouflon) 20. Armenian Mouflon Ovis gmelini gmelinii (aka Transcaucasus or Asiatic Mouflon, regionally as Arak Sheep) 21. Esfahan Mouflon Ovis gmelini isphahanica (aka Isfahan Mouflon) 22. Larestan Mouflon Ovis gmelini laristanica (aka Laristan Mouflon) URIALS 23. Transcaspian Urial Ovis vignei arkal (Depending on locality aka Kopet Dagh, Ustyurt & Turkmen Urial) 24. Bukhara Urial Ovis vignei bocharensis 25. Afghan Urial Ovis vignei cycloceros 26. -

Royle Safaris Sichuan Mammals Tour Trip Report
In March 2019 Royle Safaris ran our second specialist Sichuan Mammals Tour with a focus on a particularly special species. The trip was run with Martin Royle, Roland Zeidler & Sid Francis as our guides. We visited 3 different locations (covering the rugged bamboo forests of the greater Wolong ecosystem, the high altitude grasslands of Rouergai and the wonderful forests of Tangjiahe. We were very successful with sightings of 44 different species of mammals and over 100 species of birds including Giant Panda, Red Panda, Pallas’s Cat, Chinese Mountain Cat, Indochinese Leopard Cat, Particoloured Flying Squirrel, Golden Snub-nosed Monkey, Chinese Ferret Badger, Eurasian Otter and Chinese Pipistrelle. We ran a second Sichuan’s Mammals Tour (back to back with this one) in April 2019 and we had even more success in some areas. The sightings log for that trip will follow in a few days. We have started to promote our 2020 Sichuan Mammals Tour (with special focus on a particular special species for half of the trip); we have already received many bookings on these two trips. Our first tour for 2020 (9th – 22nd March 2020) has just one place remaining and our second tour for 2020 (25th April – 8th May 2020) which also has only one place remaining. We have also started offering places on another specialist mammal tour of China, visiting Qinghai and the wonderful Valley of the Cats. This tour is for July 2020 (1st – 15th July 2020) and focuses on Snow leopards, Eurasian lynx, Himalayan wolf, Himalayan brown bear, Tibetan antelope, Wild Yak, White-lipped deer, Alpine musk deer, Glover’s pika, Bharal, McNeil’s deer and many more species. -

Redalyc.ECOLOGICAL STRATEGIES and IMPACT of WILD BOAR IN
Mastozoología Neotropical ISSN: 0327-9383 [email protected] Sociedad Argentina para el Estudio de los Mamíferos Argentina Cuevas, M. Fernanda; Ojeda, Ricardo A.; Jaksic, Fabian M. ECOLOGICAL STRATEGIES AND IMPACT OF WILD BOAR IN PHYTOGEOGRAPHIC PROVINCES OF ARGENTINA WITH EMPHASIS ON ARIDLANDS Mastozoología Neotropical, vol. 23, núm. 2, 2016, pp. 239-254 Sociedad Argentina para el Estudio de los Mamíferos Tucumán, Argentina Available in: http://www.redalyc.org/articulo.oa?id=45750282004 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Mastozoología Neotropical, 23:239-254, Mendoza, 2016 Copyright ©SAREM, 2016 http://www.sarem.org.ar Versión impresa ISSN 0327-9383 http://www.sbmz.com.br Versión on-line ISSN 1666-0536 Sección Especial MAMÍFEROS EXÓTICOS INVASORES ECOLOGICAL STRATEGIES AND IMPACT OF WILD BOAR IN PHYTOGEOGRAPHIC PROVINCES OF ARGENTINA WITH EMPHASIS ON ARIDLANDS M. Fernanda Cuevas1, Ricardo A. Ojeda1 and Fabian M. Jaksic2 1 Grupo de Investigaciones de la Biodiversidad (GiB), IADIZA, CCT Mendoza CONICET, CC 507, 5500 Mendoza, Argentina. [Correspondence: <[email protected]>]. 2 Centro de Ecología Aplicada y Sustentabilidad (CAPES), Pontificia Universidad Católica de Chile, Casilla 114-D, Santiago, Chile ABSTRACT. Wild boar is an invasive species introduced to Argentina for sport hunting purposes. Here, this spe- cies is present in at least 8 phytogeographic provinces but we only have information in four of them (Pampean grassland, Espinal, Subantarctic and Monte Desert). We review the ecological strategies and impact of wild boar on ecosystem processes in these different phytogeographic provinces and identify knowledge gaps and research priorities for a better understanding of this invasive species in Argentina. -

Photoperiodic Properties of Circadian Rhythm in Rat
Photoperiodic properties of circadian rhythm in rat by Liang Samantha Zhang A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Neuroscience) in The University of Michigan 2011 Doctoral Committee: Associate Professor Jimo Borjigin, Chair Professor Theresa M. Lee Professor William Michael King Associate Professor Daniel Barclay Forger Assistant Professor Jiandie Lin © Liang Samantha Zhang 2011 To my loving grandparents, YaoXiang Zhang and AnNa Yu ii Acknowledgements To all who have played a role in my life these past four years, I give my thanks. First of all, I give my gratitude to the members of Borjigin Lab. To my mentor Dr. Jimo Borjigin whose intelligence and accessibility has carried me through in this journey within the circadian field. To Dr. Tiecheng Liu, who taught me all the technical knowledge necessary to perform the work presented in this dissertation, and whose surgical skills are second to none. To all the undergrads I have trained over the years, namely Abeer, Natalie, Christof, Tara, and others, whose combined hundreds if not thousands of hours in manually analyzing melatonin data have been an indispensible asset to myself and the lab. To Michelle and Ricky for taking care of all the animals over the years, which has made life much easier for the rest of us. To Alexandra, who was willing to listen and share her experiences, and to Sean, who has been a good friend both in and out of the lab. I would also like to thank my committee members for their help and support over the years. -

For Review Only 440 IUCN SSC Antelope Specialist Group
Manuscripts submitted to Journal of Mammalogy A Pliocene hybridisation event reconciles incongruent mitochondrial and nuclear gene trees among spiral-horned antelopes Journal:For Journal Review of Mammalogy Only Manuscript ID Draft Manuscript Type: Feature Article Date Submitted by the Author: n/a Complete List of Authors: Rakotoarivelo, Andrinajoro; University of Venda, Department of Zoology O'Donoghue, Paul; Specialist Wildlife Services, Specialist Wildlife Services Bruford, Michael; Cardiff University, Cardiff School of Biosciences Moodley, Yoshan; University of Venda, Department of Zoology adaptive radiation, interspecific hybridisation, paleoclimate, Sub-Saharan Keywords: Africa, Tragelaphus https://mc.manuscriptcentral.com/jmamm Page 1 of 34 Manuscripts submitted to Journal of Mammalogy 1 Yoshan Moodley, Department of Zoology, University of Venda, 2 [email protected] 3 Interspecific hybridization in Tragelaphus 4 A Pliocene hybridisation event reconciles incongruent mitochondrial and nuclear gene 5 trees among spiral-horned antelopes 6 ANDRINAJORO R. RAKATOARIVELO, PAUL O’DONOGHUE, MICHAEL W. BRUFORD, AND * 7 YOSHAN MOODLEY 8 Department of Zoology, University of Venda, University Road, Thohoyandou 0950, Republic 9 of South Africa (ARR, ForYM) Review Only 10 Specialist Wildlife Services, 102 Bowen Court, St Asaph, LL17 0JE, United Kingdom (PO) 11 Cardiff School of Biosciences, Sir Martin Evans Building, Cardiff University, Museum 12 Avenue, Cardiff, CF10 3AX, United Kingdom (MWB) 13 Natiora Ahy Madagasikara, Lot IIU57K Bis, Ampahibe, Antananarivo 101, Madagascar 14 (ARR) 15 16 1 https://mc.manuscriptcentral.com/jmamm Manuscripts submitted to Journal of Mammalogy Page 2 of 34 17 ABSTRACT 18 The spiral-horned antelopes (Genus Tragelaphus) are among the most phenotypically diverse 19 of all large mammals, and evolved in Africa during an adaptive radiation that began in the late 20 Miocene, around 6 million years ago.