Effect of Host Plants on the Development, Survivorship, and Reproduction Ofpseudococcus Viburni (Hemiptera: Pseudococcidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abiotic and Biotic Pest Refuges Hamper Biological Control of Mealybugs in California Vineyards K.M
____________________________________ Abiotic and biotic pest refuges in California vineyards 389 ABIOTIC AND BIOTIC PEST REFUGES HAMPER BIOLOGICAL CONTROL OF MEALYBUGS IN CALIFORNIA VINEYARDS K.M. Daane,1 R. Malakar-Kuenen,1 M. Guillén,2 W.J. Bentley3, M. Bianchi,4 and D. González,2 1 Division of Insect Biology, University of California, Berkeley, California, U.S.A. 2 Department of Entomology, University of California, Riverside, California, U.S.A. 3 University of California Statewide IPM Program, Kearney Agricultural Center, Parlier, California, U.S.A. 4 University of California Cooperative Extension, San Luis Obispo, California, U.S.A. INTRODUCTION Four mealybug species cause economic damage in California vineyards. These are the grape mealy- bug, Pseudococcus maritimus (Ehrhorn); obscure mealybug, Pseudococcus viburni (Signoret); longtailed mealybug, Pseudococcus longispinus (Targioni-Tozzeti); and vine mealybug, Planococcus ficus (Signoret) (Godfrey et al., 2002). The grape, obscure, and longtailed mealybugs belong to the Pseudococcus maritimus-malacearum complex–a taxonomically close group of mealybugs (Wilkey and McKenzie, 1961). However, while the origins of the grape and longtailed mealybugs are believed to be in North America, the ancestral lines of the obscure mealybug are unclear. Regardless, these three species have been known as pests in North America for nearly 100 years. The vine mealybug, in contrast, was first identified in California in the Coachella Valley in the early 1990s (Gill, 1994). It has since spread into California’s San Joaquin Valley and central coast regions, with new infestations reported each year. The four species are similar in appearance; however, mealybugs in the P. maritimus- malacearum complex have longer caudal filaments than vine mealybug (Godfrey et al., 2002). -

MOLECULAR BIOLOGY and EPIDEMIOLOGY of GRAPEVINE LEAFROLL- ASSOCIATED VIRUSES by BHANU PRIYA DONDA a Dissertation Submitted in Pa
MOLECULAR BIOLOGY AND EPIDEMIOLOGY OF GRAPEVINE LEAFROLL- ASSOCIATED VIRUSES By BHANU PRIYA DONDA A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSPHY WASHINGTON STATE UNIVERSITY Department of Plant Pathology MAY 2016 © Copyright by BHANU PRIYA DONDA, 2016 All Rights Reserved THANKS Bioengineering MAY 2014 © Copyright by BHANU PRIYA DONDA, 2016 All Rights Reserved To the Faculty of Washington State University: The members of the Committee appointed to examine the dissertation of BHANU PRIYA DONDA find it satisfactory and recommend that it be accepted. Naidu A. Rayapati, Ph.D., Chair Dennis A. Johnson, Ph.D. Duroy A. Navarre, Ph.D. George J. Vandemark, Ph.D. Siddarame Gowda, Ph.D. ii ACKNOWLEDGEMENT I would like to express my respect and deepest gratitude towards my advisor and mentor, Dr. Naidu Rayapati. I am truly appreciative of the opportunity to pursue my doctoral degree under his guidance at Washington State University (WSU), a challenging and rewarding experience that I will value the rest of my life. I am thankful to my doctoral committee members: Dr. Dennis Johnson, Dr. George Vandemark, Dr. Roy Navarre and Dr. Siddarame Gowda for helpful advice, encouragement and guidance. I would like to thank Dr. Sandya R Kesoju (USDA-IAREC, Prosser, WA) and Dr. Neil Mc Roberts (University of California, Davis) for their statistical expertise, suggestions and collaborative research on the epidemiology of grapevine leafroll disease. To Dr. Gopinath Kodetham (University of Hyderabad, Hyderabad, India), thank you for believing in me and encouraging me to go the extra mile. I thank Dr. Sridhar Jarugula (Ohio State University Agricultural Research and Development Center, Wooster, University of Ohio, Ohio, USA), Dr. -

Essential Oil on the Tea Mealy Bug, Pseudococcus Viburni Sigornet (Hemiptera: Pseudococcidae)
Archives of Phytopathology and Plant Protection ISSN: 0323-5408 (Print) 1477-2906 (Online) Journal homepage: http://www.tandfonline.com/loi/gapp20 Toxicity of Artemisia annua (Asteraceae) essential oil on the tea mealy bug, Pseudococcus viburni Sigornet (Hemiptera: Pseudococcidae) Samar Ramzi, Ali Seraji, Reza Azadi Gonbad, Seyyedeh Kimia Mirhaghparast, Zahra Mojib Haghghadam & Shiva Haghighat To cite this article: Samar Ramzi, Ali Seraji, Reza Azadi Gonbad, Seyyedeh Kimia Mirhaghparast, Zahra Mojib Haghghadam & Shiva Haghighat (2018): Toxicity of Artemisia annua (Asteraceae) essential oil on the tea mealy bug, Pseudococcus viburni Sigornet (Hemiptera: Pseudococcidae), Archives of Phytopathology and Plant Protection, DOI: 10.1080/03235408.2017.1352223 To link to this article: https://doi.org/10.1080/03235408.2017.1352223 Published online: 08 Jan 2018. Submit your article to this journal View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=gapp20 Download by: [EPFL Bibliothèque] Date: 08 January 2018, At: 07:49 ARCHIVES OF PHYTOPATHOLOGY AND PLANT PROTECTION, 2018 https://doi.org/10.1080/03235408.2017.1352223 Toxicity of Artemisia annua (Asteraceae) essential oil on the tea mealy bug, Pseudococcus viburni Sigornet (Hemiptera: Pseudococcidae) Samar Ramzia, Ali Serajia, Reza Azadi Gonbada, Seyyedeh Kimia Mirhaghparastb, Zahra Mojib Haghghadamc and Shiva Haghighata aTea Research Center, Horticulture Science Research Institute, Agricultural -

An Investigation Into the Integrated Pest Management of The
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Stellenbosch University SUNScholar Repository An investigation into the integrated pest management of the obscure mealybug, Pseudococcus viburni (Signoret) (Hemiptera: Pseudococcidae), in pome fruit orchards in the Western Cape Province, South Africa. Pride Mudavanhu Thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Agriculture (Entomology), in the Faculty of AgriSciences. at the University of Stellenbosch Supervisor: Dr Pia Addison Department of Conservation Ecology and Entomology Faculty of AgriSciences University of Stellenbosch South Africa December 2009 DECLARATION By submitting this dissertation electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the owner of the copyright thereof (unless to the extent explicitly otherwise stated) and that I have not previously in its entirety or in part submitted it for obtaining any qualification. December 2009 Copyright © 2009 Stellenbosch University All rights reserved i ABSTRACT Pseudococcus viburni (Signoret) (Hemiptera: Pseudococcidae) (obscure mealybug), is a common and serious pest of apples and pears in South Africa. Consumer and regulatory pressure to produce commodities under sustainable and ecologically compatible conditions has rendered chemical control options increasingly limited. Information on the seasonal occurrence of pests is but one of the vital components of an effective and sustainable integrated pest management system needed for planning the initiation of monitoring and determining when damage can be expected. It is also important to identify which orchards are at risk of developing mealybug infestations while development of effective and early monitoring tools for mealybug populations will help growers in making decisions with regards to pest management and crop suitability for various markets. -

A Parasitoid of Pseudococcus Viburni (Signoret) (Hemiptera: Pseudococcidae)
Revista Brasileira de Entomologia 61 (2017) 257–261 REVISTA BRASILEIRA DE Entomologia A Journal on Insect Diversity and Evolution www.rbentomologia.com Biology, Ecology and Diversity Biology of Blepyrus clavicornis (Compere) (Hymenoptera: Encyrtidae), a parasitoid of Pseudococcus viburni (Signoret) (Hemiptera: Pseudococcidae) a,b,∗ a b Vitor Pacheco da Silva , Mauro Garcia , Marcos Botton a Universidade Federal de Pelotas, Pós-Graduac¸ ão em Fitossanidade, Departamento de Fitossanidade, Pelotas, RS, Brazil b Embrapa Uva e Vinho, Bento Gonc¸ alves, RS, Brazil a b s t r a c t a r t i c l e i n f o Article history: Encyrtids (Hymenoptera: Encyrtidae) are the most important and diverse group of natural enemies of Received 28 November 2016 mealybugs (Hemiptera: Pseudococcidae). Blepyrus clavicornis (Compere) is the most common parasitoid Accepted 24 May 2017 associated with Pseudococcus viburni (Signoret) in the Serra Gaúcha region, Brazil. We conducted labora- Available online 7 June 2017 tory studies to assess the development time, sex ratio, adult longevity, host stage selection for parasitism, Associate Editor: Adeney de Freitas Bueno and effect of food on the longevity of adult females of B. clavicornis. The experiments were conducted in a ◦ climate chamber at 25 ± 1 C, 70 ± 10% RH and 12:12 L:D photoperiod. The solitary parasitoid B. clavicor- Keywords: nis parasitized third-instar and adult female stages of P. viburni. The development time was more than Biological control 30 days (31.75 ± 0.38 for females and 30.02 ± 0.34 for males) when B. clavicornis laid eggs in adult mealy- Chalcidoidea bug females, and 35 days (36.50 ± 0.50 for females and 34.24 ± 0.43 for males) on third-instar mealybug Obscure mealybug Parasitism nymphs. -

1.6 Parasitoids of Giant Whitefly
UC Riverside UC Riverside Electronic Theses and Dissertations Title Life Histories and Host Interaction Dynamics of Parasitoids Used for Biological Control of Giant Whitefly (Aleurodicus dugesii) Cockerell (Hemiptera: Aleyrodidae) Permalink https://escholarship.org/uc/item/8020w7rd Author Schoeller, Erich Nicholas Publication Date 2018 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA RIVERSIDE Life Histories and Host Interaction Dynamics of Parasitoids Used for Biological Control of Giant Whitefly (Aleurodicus dugesii) Cockerell (Hemiptera: Aleyrodidae) A Dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Entomology by Erich Nicholas Schoeller March 2018 Dissertation Committee: Dr. Richard Redak, Chairperson Dr. Timothy Paine. Dr. Matthew Daugherty Copyright by Erich Nicholas Schoeller 2018 The Dissertation of Erich Nicholas Schoeller is approved: Committee Chairperson University of California, Riverside Acknowledgements This dissertation was made possible with the kind support and help of many individuals. I would like to thank my advisors Drs. Richard Redak, Timothy Paine, and Matthew Daugherty for their wisdom and guidance. Their insightful comments and questions helped me become a better scientist and facilitated the development of quality research. I would particularly like to thank Dr. Redak for his endless patience and unwavering support throughout my degree. I wish to also thank Tom Prentice and Rebeccah Waterworth for their support and companionship. Their presence in the Redak Lab made my time there much more enjoyable. I would like to thank all of the property owners who kindly allowed me to work on their lands over the years, as well as the many undergraduate interns who helped me collect and analyze data from the experiments in this dissertation. -

Biodiversity of Natural Enemies of Pseudococcidae in the Semiarid Region of Brazil
Journal of Agricultural Science; Vol. 12, No. 7; 2020 ISSN 1916-9752 E-ISSN 1916-9760 Published by Canadian Center of Science and Education Biodiversity of Natural Enemies of Pseudococcidae in the Semiarid Region of Brazil Maria G. R. Sá1, José E. M. Oliveira2, Valmir A. Costa3 & Paulo R. C. Lopes2 1 Agricultural Sciences Campus, Federal University of São Francisco Valley, Petrolina, Pernambuco State, Brazil 2 Embrapa Semiárido, Petrolina State, Brazil 3 Biological Institute, Campinas, São Paulo, Brazil Correspondence: Maria G. R. Sá, Agricultural Sciences Campus, Federal University of São Francisco Valley, Highway BR 407, 12 Lot 543, Nilo Coelho Irrigation Project, S/N C1, Petrolina, Pernambuco State, Brazil. Tel: 55-87-99618-4185. E-mail: [email protected] Received: February 10, 2020 Accepted: May 4, 2020 Online Published: June 15, 2020 doi:10.5539/jas.v12n7p24 URL: https://doi.org/10.5539/jas.v12n7p24 Abstract Pseudococcidae species, also known as mealybugs, comprises a complex of pests in various cultivated and non-cultivated plant species, among which fruit plants are most affected by both direct and indirect damage. The incidence of these pest species has been constant in productive environments, causing significant losses due to sap suction, virus transmission, and damage to fruit quality. Thus, this study aimed to know and investigate the population dynamics of natural enemies of Pseudococcidae in different fruit plants cultivated in the semiarid region of Brazil. For this, mealybugs associated with roots, stems, leaves, and fruits of vine, pear, apple, persimmon, guava, and acerola trees were collected biweekly in 14 properties in the São Francisco Valley region, from July 2016 to June 2017. -
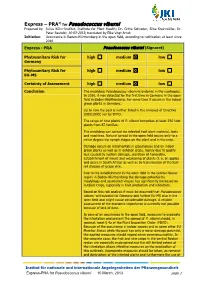
Express PRA Pseudococcus Viburni
1) Express – PRA for Pseudococcus viburni Prepared by: Julius Kühn-Institut, Institute for Plant Health; Dr. Gritta Schrader, Silke Steinmöller, Dr. Peter Baufeld; 10-03-2013; translated by Elke Vogt-Arndt Initiation: Occurrence in Baden-Württemberg in the open field, according to notification at least since 2010 Express - PRA Pseudococcus viburni (Signoret) Phytosanitary Risk for high medium low Germany Phytosanitary Risk for high medium low EU-MS Certainty of Assessment high medium low Conclusion The mealybug Pseudococcus viburni is endemic in the neotropics. In 2010, it was detected for the first time in Germany in the open field in Baden-Württemberg. For some time it occurs in the indoor green plants in Germany. Up to now the pest is neither listed in the annexes of Directive 2000/29/EC nor by EPPO. The range of host plants of P. viburni comprises at least 296 host plants from 87 families. The mealybug can spread via infested host plant material, tools and machines. Natural spread in the open field occurs only to a minor degree via nymph stages on the plant and in the crop. Damage occurs on ornamentals in glasshouses and on indoor green plants as well as in outdoor crops, mainly due to quality loss caused by suction damage, secretion of honeydew, establishment of mould and weakening of plants (f. e. on apples and pears in South Africa) as well as by transmission of the leaf- roll disease of grape vine. Due to the establishment in the open field in the central Neckar region in Baden-Württemberg the damage potential by mealybugs and associated viruses has significantly increased on outdoor crops, especially in fruit production and viticulture. -

An Investigation Into the Integrated Pest Management Of
An investigation into the integrated pest management of the obscure mealybug, Pseudococcus viburni (Signoret) (Hemiptera: Pseudococcidae), in pome fruit orchards in the Western Cape Province, South Africa. Pride Mudavanhu Thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Agriculture (Entomology), in the Faculty of AgriSciences. at the University of Stellenbosch Supervisor: Dr Pia Addison Department of Conservation Ecology and Entomology Faculty of AgriSciences University of Stellenbosch South Africa December 2009 DECLARATION By submitting this dissertation electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the owner of the copyright thereof (unless to the extent explicitly otherwise stated) and that I have not previously in its entirety or in part submitted it for obtaining any qualification. December 2009 Copyright © 2009 Stellenbosch University All rights reserved i ABSTRACT Pseudococcus viburni (Signoret) (Hemiptera: Pseudococcidae) (obscure mealybug), is a common and serious pest of apples and pears in South Africa. Consumer and regulatory pressure to produce commodities under sustainable and ecologically compatible conditions has rendered chemical control options increasingly limited. Information on the seasonal occurrence of pests is but one of the vital components of an effective and sustainable integrated pest management system needed for planning the initiation of monitoring and determining when damage can be expected. It is also important to identify which orchards are at risk of developing mealybug infestations while development of effective and early monitoring tools for mealybug populations will help growers in making decisions with regards to pest management and crop suitability for various markets. -

Documentation of Grapevine Leafroll-Associated Viruses in Wine Grape Varieties and Native Grape Species in Virginia, and Examina
Documentation of grapevine leafroll-associated viruses in wine grape varieties and native grape species in Virginia, and examination of the movement of grapevine leafroll disease to develop management strategies Taylor Jones Thesis submitted to the faculty of Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Master of Science In Plant Pathology, Physiology, and Weed Science Mizuho Nita, Chair Anton Baudoin Sue Tolin Elizabeth Bush December 4, 2012 Blacksburg, Virginia Keywords: Grapevine leafroll disease; GLRaV-2; GLRaV-3; Grapevine Fleck Virus; Mealybugs; Grapevines; Virginia Documentation of Grapevine leafroll-associated viruses in wine grape varieties and native grape species in Virginia, and examination of the movement of the grapevine leafroll disease to develop management strategies Taylor Jones ABSTRACT Grapevine leafroll-associated virus-2 (GLRaV-2), GLRaV-3, and grapevine fleck virus (GFkV) are widespread in grapes around the world. These viruses can cause significant crop loss and affect wine quality by reducing sugar accumulation and compromising skin color. Mealybugs are vectors of grapevine leafroll-associated viruses (GLRaVs). A statewide survey of commercial and wild grapevines in Virginia was conducted during 2009 through 2011. Also, vector management options were tested in two field studies. GLRaV-2, GLRaV-3, and GFkV were detected in 8%, 25%, and 1%, respectively, of over 1,200 vine samples (41 wine grape varieties) from 77 locations, and 64% of vineyards were positive for at least one of the tested viruses. All 100 wild grapevines tested were free of these three viruses, indicating that they are not alternative hosts. The majority of infected vines from commercial vineyards were planted prior to the 1990’s; however, some new plantings were also found to be positive, indicating movement of the viruses among vineyards and also potential infection prior to planting. -

Effects of Artemisia Annua Methanolic Extract on the Enzymatic Components of Intermediary Metabolism and the Antioxidant System of Pseudococcus Viburni Signoret
Journal of Plant Protection Research ISSN 1427-4345 ORIGINAL ARTICLE Effects of Artemisia annua methanolic extract on the enzymatic components of intermediary metabolism and the antioxidant system of Pseudococcus viburni Signoret Samar Ramzi1*, Ali Seraji1, Reza Azadi Gonbad1, Kimia Mirhaghparast2, Zahra Mojib-Haghghadam3 1 Tea Research Center, Horticulture Science Research Institute, Agricultural Research, Education and Extension Organization (AREEO), Lahijan, Iran 2 Department of Plant Protection, Faculty of Agricultural Sciences, University of Guilan, Rasht, Iran 3 Plant Protection Department, Research Center for Agriculture and Natural Resources, Agricultural Research, Education and Extension Organization (AREEO), Rasht, Iran Vol. 58, No. 3: 289–296, 2018 Abstract Toxicity and physiological alterations were determined in Pseudococcus viburni nymphs DOI: 10.24425/jppr.2018.124637 treated with Artemisia annua methanolic extract. The leaf dipping bioassay showed LC50 values of 0.287% and 0.194% 24 and 48 hours post-exposure. Activities of general este- Received: February 4, 2018 Accepted: September 14, 2018 rases were significantly higher in the control nymphs than in those which had been treat- ed except for the 48 h time interval using α-naphtyl acetate. The activity of glutathione *Corresponding address: S-transferase using CDNB (1-chloro-2,4-dinitrobenzene) in the control nymphs, was signif- [email protected] icantly higher than in the control at both time intervals while no significant difference was observed after 24 h in addition to the higher enzymatic activity in the treated nymphs after 48 h. All three aminotransferases were significantly more active in the control nymphs ex- cept for time intervals of 24 h for γ-glutamyl transferase and 48 h for alanine aminotrans- ferase. -

Environmental Risk Management Authority Decision
ENVIRONMENTAL RISK MANAGEMENT AUTHORITY DECISION Application code NOR99001 Application type Release from containment any New Organism under section 34(1)(b) of the Hazardous Substances and New Organisms (HSNO) Act 1996. Applicant Hawke‟s Bay Pipfruit IFP Group 20 Organism Pseudaphycus maculipennis (Mercet 1923) (Hymenoptera: Encyrtidae) Purpose To release from containment the insect Pseudaphycus maculipennis for biological control of the obscure mealybug Pseudococcus viburni (Signoret 1875) (Hemiptera: Pseudococcidae). Date application received 16 April 1999 Hearing date 09 May 2000 Considered by A Special Committee of the Authority appointed under section 19(2)(b) of the HSNO Act 1996. ERMA New Zealand Anne Rose contact Decision The application to release from containment the new organism, Pseudaphycus maculipennis (Mercet 1923) (Hymenoptera: Encyrtidae) is granted in accordance with section 38(1)(a) of the HSNO Act. As required under section 38(2), there are no controls on this approval. Purpose of Application The application is for approval to release from containment the insect parasitoid P. maculipennis, for biological control of obscure mealybug, P. viburni. P. viburni is a polyphagous, cosmopolitan pest with a worldwide distribution. P. viburni was first identified in New Zealand in 1922 and has been a significant pest of pipfruit, particularly in Hawke‟s Bay, since the late 1960s. P. viburni is difficult for pipfruit growers to control because it is cryptic in nature, infesting the calyx of fruit, which makes it difficult to achieve adequate spray coverage. The honeydew produced by P. viburni leads to the development of sooty mould, making fruit unsaleable. Lesser levels of infestation create grading and packing difficulties for export packhouses.