A Parasitoid of Pseudococcus Viburni (Signoret) (Hemiptera: Pseudococcidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abiotic and Biotic Pest Refuges Hamper Biological Control of Mealybugs in California Vineyards K.M
____________________________________ Abiotic and biotic pest refuges in California vineyards 389 ABIOTIC AND BIOTIC PEST REFUGES HAMPER BIOLOGICAL CONTROL OF MEALYBUGS IN CALIFORNIA VINEYARDS K.M. Daane,1 R. Malakar-Kuenen,1 M. Guillén,2 W.J. Bentley3, M. Bianchi,4 and D. González,2 1 Division of Insect Biology, University of California, Berkeley, California, U.S.A. 2 Department of Entomology, University of California, Riverside, California, U.S.A. 3 University of California Statewide IPM Program, Kearney Agricultural Center, Parlier, California, U.S.A. 4 University of California Cooperative Extension, San Luis Obispo, California, U.S.A. INTRODUCTION Four mealybug species cause economic damage in California vineyards. These are the grape mealy- bug, Pseudococcus maritimus (Ehrhorn); obscure mealybug, Pseudococcus viburni (Signoret); longtailed mealybug, Pseudococcus longispinus (Targioni-Tozzeti); and vine mealybug, Planococcus ficus (Signoret) (Godfrey et al., 2002). The grape, obscure, and longtailed mealybugs belong to the Pseudococcus maritimus-malacearum complex–a taxonomically close group of mealybugs (Wilkey and McKenzie, 1961). However, while the origins of the grape and longtailed mealybugs are believed to be in North America, the ancestral lines of the obscure mealybug are unclear. Regardless, these three species have been known as pests in North America for nearly 100 years. The vine mealybug, in contrast, was first identified in California in the Coachella Valley in the early 1990s (Gill, 1994). It has since spread into California’s San Joaquin Valley and central coast regions, with new infestations reported each year. The four species are similar in appearance; however, mealybugs in the P. maritimus- malacearum complex have longer caudal filaments than vine mealybug (Godfrey et al., 2002). -

MOLECULAR BIOLOGY and EPIDEMIOLOGY of GRAPEVINE LEAFROLL- ASSOCIATED VIRUSES by BHANU PRIYA DONDA a Dissertation Submitted in Pa
MOLECULAR BIOLOGY AND EPIDEMIOLOGY OF GRAPEVINE LEAFROLL- ASSOCIATED VIRUSES By BHANU PRIYA DONDA A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSPHY WASHINGTON STATE UNIVERSITY Department of Plant Pathology MAY 2016 © Copyright by BHANU PRIYA DONDA, 2016 All Rights Reserved THANKS Bioengineering MAY 2014 © Copyright by BHANU PRIYA DONDA, 2016 All Rights Reserved To the Faculty of Washington State University: The members of the Committee appointed to examine the dissertation of BHANU PRIYA DONDA find it satisfactory and recommend that it be accepted. Naidu A. Rayapati, Ph.D., Chair Dennis A. Johnson, Ph.D. Duroy A. Navarre, Ph.D. George J. Vandemark, Ph.D. Siddarame Gowda, Ph.D. ii ACKNOWLEDGEMENT I would like to express my respect and deepest gratitude towards my advisor and mentor, Dr. Naidu Rayapati. I am truly appreciative of the opportunity to pursue my doctoral degree under his guidance at Washington State University (WSU), a challenging and rewarding experience that I will value the rest of my life. I am thankful to my doctoral committee members: Dr. Dennis Johnson, Dr. George Vandemark, Dr. Roy Navarre and Dr. Siddarame Gowda for helpful advice, encouragement and guidance. I would like to thank Dr. Sandya R Kesoju (USDA-IAREC, Prosser, WA) and Dr. Neil Mc Roberts (University of California, Davis) for their statistical expertise, suggestions and collaborative research on the epidemiology of grapevine leafroll disease. To Dr. Gopinath Kodetham (University of Hyderabad, Hyderabad, India), thank you for believing in me and encouraging me to go the extra mile. I thank Dr. Sridhar Jarugula (Ohio State University Agricultural Research and Development Center, Wooster, University of Ohio, Ohio, USA), Dr. -

Bugila Phd Thesis Document Final
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UTL Repository Host-parasitoid relationships of Anagyrus sp. near pseudococci (Girault), (Hymenoptera, Encyrtidae), as a basis to improve biological control of pest mealybugs (Hemiptera, Pseudococcidae) TESE APRESENTADA PARA OBTENÇÃO DO GRAU DE DOUTOR EM ENGENHARIA AGRONÓMICA Abdalbaset Abusalah Ali Bugila Orientador: Professor Doutor José Carlos Franco Santos Silva Co-orientador: Professora Doutora Manuela Rodrigues Branco Simões JÚRI: Presidente : Reitor da Universidade de Lisboa Vogais: Doutora Laura Monteiro Torres Professora Catedrática, Escola de Ciências Agrárias e Veterinárias da Universidade de Trás-os-Montes e Alto Douro Doutor António Maria Marques Mexia Professor Catedrático, Instituto Superior de Agronomia da Universidade de Lisboa Doutor David João Horta Lopes Professor Auxiliar com agregação, Universidade dos Açores; Doutor José Carlos Franco Santos Silva Professor Auxiliar, Instituto Superior de Agronomia da Universidade de Lisboa Doutora Elisabete Tavares Lacerda de Figueiredo Oliveira Professora Auxiliar, Instituto Superior de Agronomia da Universidade de Lisboa. LISBOA 2014 Index Abstract vii ....................................................................................................................... Resumo viii ………………………………………………………………………..….…. 1. Introduction 1 ………………………………………………………………….….. 1.1. State of the art 2 …………………………………………………………….…. 1.2. Objectives 4 …………………………………………………………………..... 1.3. References 5 ……………………………………………………………….….. -

Essential Oil on the Tea Mealy Bug, Pseudococcus Viburni Sigornet (Hemiptera: Pseudococcidae)
Archives of Phytopathology and Plant Protection ISSN: 0323-5408 (Print) 1477-2906 (Online) Journal homepage: http://www.tandfonline.com/loi/gapp20 Toxicity of Artemisia annua (Asteraceae) essential oil on the tea mealy bug, Pseudococcus viburni Sigornet (Hemiptera: Pseudococcidae) Samar Ramzi, Ali Seraji, Reza Azadi Gonbad, Seyyedeh Kimia Mirhaghparast, Zahra Mojib Haghghadam & Shiva Haghighat To cite this article: Samar Ramzi, Ali Seraji, Reza Azadi Gonbad, Seyyedeh Kimia Mirhaghparast, Zahra Mojib Haghghadam & Shiva Haghighat (2018): Toxicity of Artemisia annua (Asteraceae) essential oil on the tea mealy bug, Pseudococcus viburni Sigornet (Hemiptera: Pseudococcidae), Archives of Phytopathology and Plant Protection, DOI: 10.1080/03235408.2017.1352223 To link to this article: https://doi.org/10.1080/03235408.2017.1352223 Published online: 08 Jan 2018. Submit your article to this journal View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=gapp20 Download by: [EPFL Bibliothèque] Date: 08 January 2018, At: 07:49 ARCHIVES OF PHYTOPATHOLOGY AND PLANT PROTECTION, 2018 https://doi.org/10.1080/03235408.2017.1352223 Toxicity of Artemisia annua (Asteraceae) essential oil on the tea mealy bug, Pseudococcus viburni Sigornet (Hemiptera: Pseudococcidae) Samar Ramzia, Ali Serajia, Reza Azadi Gonbada, Seyyedeh Kimia Mirhaghparastb, Zahra Mojib Haghghadamc and Shiva Haghighata aTea Research Center, Horticulture Science Research Institute, Agricultural -

Morphology and Distribution of Antennal Sensilla in a Mealybug Parasitoid, Anagyrus Sp
8 Microsc. Microanal. 21 (Suppl 6), 2015 doi:10.1017/S1431927614013713 © Microscopy Society of America 2015 Morphology and distribution of antennal sensilla in a mealybug parasitoid, Anagyrus sp. near pseudococci (Hymenoptera, Encyrtidae) Fortuna, T.M.*, Franco, J.C.** and Rebelo, M.T.*** * Department of Terrestrial Ecology, Netherlands Institute of Ecology (NIOO-KNAW), 6700 AB Wageningen, The Netherlands ** Centro de Estudos Florestais, Instituto Superior de Agronomia, Universidade Técnica de Lisboa, 1349-017 Lisboa, Portugal *** Departamento de Biologia Animal (DBA)/Centro de Estudos do Ambiente e do Mar (CESAM), Faculdade de Ciências da Universidade de Lisboa, Campo Grande, 1749-016 Lisboa, Portugal E-mail: [email protected] The antennae of adult insects have various types of sensilla with different functions in the various behaviors of adult life. Parasitoid females are known to rely on these important sensory receptors in their antennae to locate and select the host [1-3]. Anagyrus sp. near pseudococci (4) is a solitary endoparasitoid of pest mealybugs (Hemiptera, Pseudococcidae), such as Planococcus ficus (Signoret) and P. citri (Risso) [5]. The females of A. sp. near pseudococci were recently shown to respond to the sex pheromone of P. ficus and use this kairomonal cue in host location [5, 6]. However, little is still known about the antennal receptors that might be involved in the parasitoid host selection behavior. In this study, using SEM (JEOL JSM 5200 LV), we describe the morphology and distribution of sensilla on the antenna of 18 females and 2 males of A. sp. near pseudococci. Eight types of antennal sensilla were found on adult wasps. -

An Investigation Into the Integrated Pest Management of The
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Stellenbosch University SUNScholar Repository An investigation into the integrated pest management of the obscure mealybug, Pseudococcus viburni (Signoret) (Hemiptera: Pseudococcidae), in pome fruit orchards in the Western Cape Province, South Africa. Pride Mudavanhu Thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Agriculture (Entomology), in the Faculty of AgriSciences. at the University of Stellenbosch Supervisor: Dr Pia Addison Department of Conservation Ecology and Entomology Faculty of AgriSciences University of Stellenbosch South Africa December 2009 DECLARATION By submitting this dissertation electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the owner of the copyright thereof (unless to the extent explicitly otherwise stated) and that I have not previously in its entirety or in part submitted it for obtaining any qualification. December 2009 Copyright © 2009 Stellenbosch University All rights reserved i ABSTRACT Pseudococcus viburni (Signoret) (Hemiptera: Pseudococcidae) (obscure mealybug), is a common and serious pest of apples and pears in South Africa. Consumer and regulatory pressure to produce commodities under sustainable and ecologically compatible conditions has rendered chemical control options increasingly limited. Information on the seasonal occurrence of pests is but one of the vital components of an effective and sustainable integrated pest management system needed for planning the initiation of monitoring and determining when damage can be expected. It is also important to identify which orchards are at risk of developing mealybug infestations while development of effective and early monitoring tools for mealybug populations will help growers in making decisions with regards to pest management and crop suitability for various markets. -

1.6 Parasitoids of Giant Whitefly
UC Riverside UC Riverside Electronic Theses and Dissertations Title Life Histories and Host Interaction Dynamics of Parasitoids Used for Biological Control of Giant Whitefly (Aleurodicus dugesii) Cockerell (Hemiptera: Aleyrodidae) Permalink https://escholarship.org/uc/item/8020w7rd Author Schoeller, Erich Nicholas Publication Date 2018 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA RIVERSIDE Life Histories and Host Interaction Dynamics of Parasitoids Used for Biological Control of Giant Whitefly (Aleurodicus dugesii) Cockerell (Hemiptera: Aleyrodidae) A Dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Entomology by Erich Nicholas Schoeller March 2018 Dissertation Committee: Dr. Richard Redak, Chairperson Dr. Timothy Paine. Dr. Matthew Daugherty Copyright by Erich Nicholas Schoeller 2018 The Dissertation of Erich Nicholas Schoeller is approved: Committee Chairperson University of California, Riverside Acknowledgements This dissertation was made possible with the kind support and help of many individuals. I would like to thank my advisors Drs. Richard Redak, Timothy Paine, and Matthew Daugherty for their wisdom and guidance. Their insightful comments and questions helped me become a better scientist and facilitated the development of quality research. I would particularly like to thank Dr. Redak for his endless patience and unwavering support throughout my degree. I wish to also thank Tom Prentice and Rebeccah Waterworth for their support and companionship. Their presence in the Redak Lab made my time there much more enjoyable. I would like to thank all of the property owners who kindly allowed me to work on their lands over the years, as well as the many undergraduate interns who helped me collect and analyze data from the experiments in this dissertation. -

New Controls Investigated for Vine Mealybug
RESEARCH ArticLE t of Industrial Hygienists. Lewis Pub. New controls investigated Faucett J, Meyers J, Tejeda D, et al. 2001. An instrument to measure muscu- for vine mealybug loskeletal symptoms among immigrant Hispanic farmworkers: Validation in the nursery industry. J Agric Saf Health 7(3):185–98. Kent M. Daane Garg A, Chaffin D, Herrin G. 1978. Walter J. Bentley n the early 1990s, the vine mealybug Prediction of metabolic rates for manual Vaughn M. Walton was accidentally introduced into the materials handling jobs. Am Ind Hyg Assoc J CoachellaI Valley (Gill 1994; Godfrey 39(8):661–74. Raksha Malakar-Kuenen et al. 2003), probably from Mexican or Glisan B. 1993. Customized prevention Jocelyn G. Millar programs play vital role in back protection Argentinian table-grape vineyards. This Chuck A. Ingels process. Occup Health Safety 62(12):21–6. invasive pest quickly spread to grape- Ed A. Weber Hashemi L, Webster BS, Clancy EA, growing regions in the San Joaquin Val- Volinn E. 1997. Length of disability and Carmen Gispert cost of workers’ compensation low back ley (1998), Central Coast (1999), North pain claims. J Occupational Environ Med t Coast (2001), Sacramento Valley (2002), 39(10):937–45. Sierra foothills (2002) and Monterey Leamon TB. 1994. Research to reality: A critical review of the validity of various cri- The vine mealybug is a newly inva- area (2002). As of fall 2005, the vine teria for the prevention of occupationally sive pest that has spread throughout mealybug had been found in 17 Califor- induced low back pain disability. Ergonom- California’s extensive grape-growing nia counties, and it is likely that more ics 37(12):1959–74. -

An Alternative Perspective for the Theory of Biological Control
insects Perspective An Alternative Perspective for the Theory of Biological Control Nicholas J. Mills Department of Environmental Science, Policy and Management, University of California, Berkeley, CA 94720-3114, USA; [email protected]; Tel.: +1-510-642-1711 Received: 12 September 2018; Accepted: 29 September 2018; Published: 2 October 2018 Abstract: Importation biological control represents the planned introduction of a specialist natural enemy from the region of origin of an invasive pest or weed. For this study, the author considered why attempts to develop a predictive theory for biological control have been misguided and what future directions might be more promising and effective. Despite considerable interest in the theory of consumer–resource population dynamics, such theory has contributed little to improvements in the success of biological control due to a focus on persistence and equilibrium dynamics rather than establishment and impact. A broader consideration of invasion biology in addition to population ecology offers new opportunities for a more inclusive theory of biological control that incorporates the demographic and genetic processes that more specifically address the establishment and impact of introduced natural enemies. The importance of propagule size and genetic variance for successful establishment, and of contributions to host population growth, relative population growth rates, interaction strength, and coevolution for suppression of host abundance are discussed as promising future directions for a theory of biological control. Keywords: pest; weed; importation; establishment; impact; demography; genetics 1. Introduction Biological control is an ecosystem service in which a pest or weed is effectively controlled through interactions with its natural enemies, and in many cases the natural enemies are insects [1]. -

Effect of Host Plants on the Development, Survivorship, and Reproduction Ofpseudococcus Viburni (Hemiptera: Pseudococcidae)
Effect of host plants on the development, survivorship, and reproduction of Pseudococcus viburni (Hemiptera: Pseudococcidae) Vitor Cezar Pacheco da Silva1,*, Aline Nondillo2, Elisangela Caroline Weber Galzer2, Mauro Silveira Garcia1, Marcos Botton2 Abstract The obscure mealybug Pseudococcus viburni (Signoret) (Hemiptera: Pseudococcidae) is one of the most important damaging mealybugs in vineyards worldwide and in South American temperate fruit production. The life history ofP. viburni on 3 fruit species was studied under laboratory conditions. Leaves of apple Malus domestica (Borkh.), persimmon Diospyros kaki L., and grapevine Vitis labrusca L., were used as host plants. Mealybugs were able to develop, survive, and reproduce on all 3 hosts; however, persimmon and grape leaves were most suitable for P. viburni development and reproduction. The cumulative development time (nymph to adulthood) ranged from about 35 d on persimmon and grape leaves to 40.87 ± 0.53 d on apple leaves for females, and from 30.68 ± 0.44 d on persimmon to about 34 d on apple and grape leaves for males. The obscure mealybug exhibited biparental oviparous reproduction, and the fecundity significantly decreased on apple leaves from a mean of 88 eggs per female on persimmon and grape leaves to 30.61 ± 2.9 eggs on apple leaves. The preoviposition period was shortest on persimmon (17.57 ± 0.59 d), and the reproductive period was longest on grape (31.77 ± 0.92 d). The longevity of mated females was higher on grape (54.84 ± 1.23 d) than on apple and persimmon (about 43 d). These results suggest that all 3 hosts can support the development and reproduction of P. -

Biodiversity of Natural Enemies of Pseudococcidae in the Semiarid Region of Brazil
Journal of Agricultural Science; Vol. 12, No. 7; 2020 ISSN 1916-9752 E-ISSN 1916-9760 Published by Canadian Center of Science and Education Biodiversity of Natural Enemies of Pseudococcidae in the Semiarid Region of Brazil Maria G. R. Sá1, José E. M. Oliveira2, Valmir A. Costa3 & Paulo R. C. Lopes2 1 Agricultural Sciences Campus, Federal University of São Francisco Valley, Petrolina, Pernambuco State, Brazil 2 Embrapa Semiárido, Petrolina State, Brazil 3 Biological Institute, Campinas, São Paulo, Brazil Correspondence: Maria G. R. Sá, Agricultural Sciences Campus, Federal University of São Francisco Valley, Highway BR 407, 12 Lot 543, Nilo Coelho Irrigation Project, S/N C1, Petrolina, Pernambuco State, Brazil. Tel: 55-87-99618-4185. E-mail: [email protected] Received: February 10, 2020 Accepted: May 4, 2020 Online Published: June 15, 2020 doi:10.5539/jas.v12n7p24 URL: https://doi.org/10.5539/jas.v12n7p24 Abstract Pseudococcidae species, also known as mealybugs, comprises a complex of pests in various cultivated and non-cultivated plant species, among which fruit plants are most affected by both direct and indirect damage. The incidence of these pest species has been constant in productive environments, causing significant losses due to sap suction, virus transmission, and damage to fruit quality. Thus, this study aimed to know and investigate the population dynamics of natural enemies of Pseudococcidae in different fruit plants cultivated in the semiarid region of Brazil. For this, mealybugs associated with roots, stems, leaves, and fruits of vine, pear, apple, persimmon, guava, and acerola trees were collected biweekly in 14 properties in the São Francisco Valley region, from July 2016 to June 2017. -
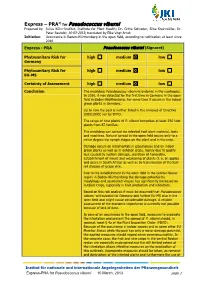
Express PRA Pseudococcus Viburni
1) Express – PRA for Pseudococcus viburni Prepared by: Julius Kühn-Institut, Institute for Plant Health; Dr. Gritta Schrader, Silke Steinmöller, Dr. Peter Baufeld; 10-03-2013; translated by Elke Vogt-Arndt Initiation: Occurrence in Baden-Württemberg in the open field, according to notification at least since 2010 Express - PRA Pseudococcus viburni (Signoret) Phytosanitary Risk for high medium low Germany Phytosanitary Risk for high medium low EU-MS Certainty of Assessment high medium low Conclusion The mealybug Pseudococcus viburni is endemic in the neotropics. In 2010, it was detected for the first time in Germany in the open field in Baden-Württemberg. For some time it occurs in the indoor green plants in Germany. Up to now the pest is neither listed in the annexes of Directive 2000/29/EC nor by EPPO. The range of host plants of P. viburni comprises at least 296 host plants from 87 families. The mealybug can spread via infested host plant material, tools and machines. Natural spread in the open field occurs only to a minor degree via nymph stages on the plant and in the crop. Damage occurs on ornamentals in glasshouses and on indoor green plants as well as in outdoor crops, mainly due to quality loss caused by suction damage, secretion of honeydew, establishment of mould and weakening of plants (f. e. on apples and pears in South Africa) as well as by transmission of the leaf- roll disease of grape vine. Due to the establishment in the open field in the central Neckar region in Baden-Württemberg the damage potential by mealybugs and associated viruses has significantly increased on outdoor crops, especially in fruit production and viticulture.