Chapter 8. the Atomic Nucleus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Five Common Particles
The Five Common Particles The world around you consists of only three particles: protons, neutrons, and electrons. Protons and neutrons form the nuclei of atoms, and electrons glue everything together and create chemicals and materials. Along with the photon and the neutrino, these particles are essentially the only ones that exist in our solar system, because all the other subatomic particles have half-lives of typically 10-9 second or less, and vanish almost the instant they are created by nuclear reactions in the Sun, etc. Particles interact via the four fundamental forces of nature. Some basic properties of these forces are summarized below. (Other aspects of the fundamental forces are also discussed in the Summary of Particle Physics document on this web site.) Force Range Common Particles It Affects Conserved Quantity gravity infinite neutron, proton, electron, neutrino, photon mass-energy electromagnetic infinite proton, electron, photon charge -14 strong nuclear force ≈ 10 m neutron, proton baryon number -15 weak nuclear force ≈ 10 m neutron, proton, electron, neutrino lepton number Every particle in nature has specific values of all four of the conserved quantities associated with each force. The values for the five common particles are: Particle Rest Mass1 Charge2 Baryon # Lepton # proton 938.3 MeV/c2 +1 e +1 0 neutron 939.6 MeV/c2 0 +1 0 electron 0.511 MeV/c2 -1 e 0 +1 neutrino ≈ 1 eV/c2 0 0 +1 photon 0 eV/c2 0 0 0 1) MeV = mega-electron-volt = 106 eV. It is customary in particle physics to measure the mass of a particle in terms of how much energy it would represent if it were converted via E = mc2. -

The Moedal Experiment at the LHC. Searching Beyond the Standard
126 EPJ Web of Conferences , 02024 (2016) DOI: 10.1051/epjconf/201612602024 ICNFP 2015 The MoEDAL experiment at the LHC Searching beyond the standard model James L. Pinfold (for the MoEDAL Collaboration)1,a 1 University of Alberta, Physics Department, Edmonton, Alberta T6G 0V1, Canada Abstract. MoEDAL is a pioneering experiment designed to search for highly ionizing avatars of new physics such as magnetic monopoles or massive (pseudo-)stable charged particles. Its groundbreaking physics program defines a number of scenarios that yield potentially revolutionary insights into such foundational questions as: are there extra dimensions or new symmetries; what is the mechanism for the generation of mass; does magnetic charge exist; what is the nature of dark matter; and, how did the big-bang develop. MoEDAL’s purpose is to meet such far-reaching challenges at the frontier of the field. The innovative MoEDAL detector employs unconventional methodologies tuned to the prospect of discovery physics. The largely passive MoEDAL detector, deployed at Point 8 on the LHC ring, has a dual nature. First, it acts like a giant camera, comprised of nuclear track detectors - analyzed offline by ultra fast scanning microscopes - sensitive only to new physics. Second, it is uniquely able to trap the particle messengers of physics beyond the Standard Model for further study. MoEDAL’s radiation environment is monitored by a state-of-the-art real-time TimePix pixel detector array. A new MoEDAL sub-detector to extend MoEDAL’s reach to millicharged, minimally ionizing, particles (MMIPs) is under study Finally we shall describe the next step for MoEDAL called Cosmic MoEDAL, where we define a very large high altitude array to take the search for highly ionizing avatars of new physics to higher masses that are available from the cosmos. -

Models of the Atomic Nucleus
Models of the Atomic Nucleus Second Edition Norman D. Cook Models of the Atomic Nucleus Unification Through a Lattice of Nucleons Second Edition 123 Prof. Norman D. Cook Kansai University Dept. Informatics 569 Takatsuki, Osaka Japan [email protected] Additional material to this book can be downloaded from http://extras.springer.com. ISBN 978-3-642-14736-4 e-ISBN 978-3-642-14737-1 DOI 10.1007/978-3-642-14737-1 Springer Heidelberg Dordrecht London New York Library of Congress Control Number: 2010936431 © Springer-Verlag Berlin Heidelberg 2006, 2010 This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilm or in any other way, and storage in data banks. Duplication of this publication or parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965, in its current version, and permission for use must always be obtained from Springer. Violations are liable to prosecution under the German Copyright Law. The use of general descriptive names, registered names, trademarks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. Cover design: WMXDesign GmbH, Heidelberg Printed on acid-free paper Springer is part of Springer Science+Business Media (www.springer.com) Preface to the Second Edition Already by the 1970s, some theorists had declared that nuclear structure physics was a “closed chapter” in science, but since then it has repeatedly been found necessary to re-open this closed chapter to address old problems and to explain new phenom- ena. -

CHAPTER 12: the Atomic Nucleus
CHAPTER 12 The Atomic Nucleus ◼ 12.1 Discovery of the Neutron ◼ 12.2 Nuclear Properties ◼ 12.3 The Deuteron ◼ 12.4 Nuclear Forces ◼ 12.5 Nuclear Stability ◼ 12.6 Radioactive Decay ◼ 12.7 Alpha, Beta, and Gamma Decay ◼ 12.8 Radioactive Nuclides Structure of matter Dark matter and dark energy are the yin and yang of the cosmos. Dark matter produces an attractive force (gravity), while dark energy produces a repulsive force (antigravity). ... Astronomers know dark matter exists because visible matter doesn't have enough gravitational muster to hold galaxies together. Hierarchy of forces ◼ Sta Standard Model tries to unify the forces into one force Ernest Rutherford “Father of the Nucleus” Story so far: Unification Faraday Glashow,Weinberg,Salam Georgi,Glashow Green,Schwarz Witten 1831 1967 1974 1984 1995 Electricity } } } Electromagnetic force } } } Magnetism} } Electro-weak force } } } Weak nuclear force} } Grand unified force } } } 5 Different } Strong nuclear force} } D=10 String } M-theory ? } Theories } Gravitational force} +branes in D=11 Page 6 © Imperial College London Discovery of the Neutron 3) Nuclear magnetic moment: The magnetic moment of an electron is over 1000 times larger than that of a proton. The measured nuclear magnetic moments are on the same order of magnitude as the proton’s, so an electron is not a part of the nucleus. ◼ In 1930 the German physicists Bothe and Becker used a radioactive polonium source that emitted α particles. When these α particles bombarded beryllium, the radiation penetrated several centimeters of lead. The neutrons collide elastically with the protons of the paraffin thereby producing the5.7 MeV protons Discovery of the Neutron ◼ Photons are called gamma rays when they originate from the nucleus. -

Non-Collider Searches for Stable Massive Particles
Non-collider searches for stable massive particles S. Burdina, M. Fairbairnb, P. Mermodc,, D. Milsteadd, J. Pinfolde, T. Sloanf, W. Taylorg aDepartment of Physics, University of Liverpool, Liverpool L69 7ZE, UK bDepartment of Physics, King's College London, London WC2R 2LS, UK cParticle Physics department, University of Geneva, 1211 Geneva 4, Switzerland dDepartment of Physics, Stockholm University, 106 91 Stockholm, Sweden ePhysics Department, University of Alberta, Edmonton, Alberta, Canada T6G 0V1 fDepartment of Physics, Lancaster University, Lancaster LA1 4YB, UK gDepartment of Physics and Astronomy, York University, Toronto, ON, Canada M3J 1P3 Abstract The theoretical motivation for exotic stable massive particles (SMPs) and the results of SMP searches at non-collider facilities are reviewed. SMPs are defined such that they would be suffi- ciently long-lived so as to still exist in the cosmos either as Big Bang relics or secondary collision products, and sufficiently massive such that they are typically beyond the reach of any conceiv- able accelerator-based experiment. The discovery of SMPs would address a number of important questions in modern physics, such as the origin and composition of dark matter and the unifi- cation of the fundamental forces. This review outlines the scenarios predicting SMPs and the techniques used at non-collider experiments to look for SMPs in cosmic rays and bound in mat- ter. The limits so far obtained on the fluxes and matter densities of SMPs which possess various detection-relevant properties such as electric and magnetic charge are given. Contents 1 Introduction 4 2 Theory and cosmology of various kinds of SMPs 4 2.1 New particle states (elementary or composite) . -

Lesson 6.3 You Experience Is Related to Your Location on the Globe
Key Objectives 6.3.1 DESCRIBE trends among elements for 6.3 Periodic Trends atomic size. 6.3.2 EXPLAIN how ions form. 6.3.3 DESCRIBE trends for first ionization energy, ionic size, and electronegativity. CHEMISTRY & YOUY Additional Resources Q: How are trends in the weather similar to trends in the properties of elements? Although the weather changes from day to day. The weather Reading and Study Workbook, Lesson 6.3 you experience is related to your location on the globe. For example, LESSON 6.3 Available Online or on Digital Media: Florida has an average temperature that is higher than Minnesota’s. Similarly, a rain forest receives more rain than a desert. These differ- • Teaching Resources, Lesson 6.3 Review ences are attributable to trends in the weather. In this lesson, you will • Small-Scale Chemistry Laboratory Manual, Lab 9 learn how a property such as atomic size is related to the location of an element in the periodic table. Key Questions Trends in Atomic Size What are the trends among the What are the trends among the elements for atomic size? ? elements for atomic size One way to think about atomic size is to look at the units that form How do ions form? when atoms of the same element are joined to one another. These What are the trends among the units are called molecules. Figure 6.14 shows models of molecules Engage elements for first ionization energy, (molecular models) for seven nonmetals. Because the atoms in each ionic size, and electronegativity? molecule are identical, the distance between the nuclei of these atoms CHEMISTRY YOUYOY U Have students read the can be used to estimate the size of the atoms. -
Proton Therapy ACKNOWLEDGEMENTS
AMERICAN BRAIN TUMOR ASSOCIATION Proton Therapy ACKNOWLEDGEMENTS ABOUT THE AMERICAN BRAIN TUMOR ASSOCIATION Founded in 1973, the American Brain Tumor Association (ABTA) was the first national nonprofit organization dedicated solely to brain tumor research. For over 40 years, the Chicago-based ABTA has been providing comprehensive resources that support the complex needs of brain tumor patients and caregivers, as well as the critical funding of research in the pursuit of breakthroughs in brain tumor diagnosis, treatment and care. To learn more about the ABTA, visit www.abta.org. We gratefully acknowledge Anita Mahajan, Director of International Development, MD Anderson Proton Therapy Center, director, Pediatric Radiation Oncology, co-section head of Pediatric and CNS Radiation Oncology, The University of Texas MD Anderson Cancer Center; Kevin S. Oh, MD, Department of Radiation Oncology, Massachusetts General Hospital; and Sridhar Nimmagadda, PhD, assistant professor of Radiology, Medicine and Oncology, Johns Hopkins University, for their review of this edition of this publication. This publication is not intended as a substitute for professional medical advice and does not provide advice on treatments or conditions for individual patients. All health and treatment decisions must be made in consultation with your physician(s), utilizing your specific medical information. Inclusion in this publication is not a recommendation of any product, treatment, physician or hospital. COPYRIGHT © 2015 ABTA REPRODUCTION WITHOUT PRIOR WRITTEN PERMISSION IS PROHIBITED AMERICAN BRAIN TUMOR ASSOCIATION Proton Therapy INTRODUCTION Brain tumors are highly variable in their treatment and prognosis. Many are benign and treated conservatively, while others are malignant and require aggressive combinations of surgery, radiation and chemotherapy. -

Appendix: Key Concepts and Vocabulary for Nuclear Energy
Appendix: Key Concepts and Vocabulary for Nuclear Energy The likelihood of fission depends on, among other Power Plant things, the energy of the incoming neutron. Some In most power plants around the world, heat, usually nuclei can undergo fission even when hit by a low- produced in the form of steam, is converted to energy neutron. Such elements are called fissile. electricity. The heat could come through the burning The most important fissile nuclides are the uranium of coal or natural gas, in the case of fossil-fueled isotopes, uranium-235 and uranium-233, and the power plants, or the fission of uranium or plutonium plutonium isotope, plutonium-239. Isotopes are nuclei. The rate of electrical power production in variants of the same chemical element that have these power plants is usually measured in megawatts the same number of protons and electrons, but or millions of watts, and a typical large coal or nuclear differ in the number of neutrons. Of these, only power plant today produces electricity at a rate of uranium-235 is found in nature, and it is found about 1,000 megawatts. A much smaller physical only in very low concentrations. Uranium in nature unit, the kilowatt, is a thousand watts, and large contains 0.7 percent uranium-235 and 99.3 percent household appliances use electricity at a rate of a few uranium-238. This more abundant variety is an kilowatts when they are running. The reader will have important example of a nucleus that can be split only heard about the “kilowatt-hour,” which is the amount by a high-energy neutron. -
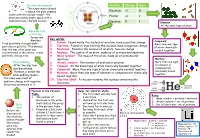
Key Words: 1. Proton: Found Inside the Nucleus of an Atom, Have a Positive Charge 2. Electron: Found in Rings Orbiting the Nucle
Nucleus development Particle Charge Mass This experiment allowed Rutherford to replace the plum pudding Electron -1 0 model with the nuclear model – the Proton +1 1 atom was mainly empty space with a small positively charged nucleus Neutron 0 1 Element All the same type of atom Alpha particle scattering Geiger and Key words: Marsden 1. Proton: Found inside the nucleus of an atom, have a positive charge Compound fired positively-charged alpha More than one type 2. Electron: Found in rings orbiting the nucleus, have a negative charge particles at gold foil. This showed of atom chemically that the mas of an atom was 3. Neutrons: Found in the nucleus of an atom, have no charge bonded together concentrated in the centre, it was 4. Nucleus: The centre of an atom, made up of protons and neutrons positively charged too 5. Mass number: The mass of the atom, made up of protons and neutrons Mixture Plum pudding 6. Atomic number: The number of protons in an atom More than one type After the electron 7. Element: All the same type of atom chemically bonded together of element or was discovered, 8. Compound: More than one type of atom chemically bonded together compound not chemically bound Thomson created the 9. Mixture: More than one type of element or compound not chemically together plum pudding model – bound together the atom was a ball of 10. Electron Shell: A ring surrounding the nucleus containing the positive charge with negative electrons electrons scattered in it Position in the Periodic Rules for electron shells: Table: 1. -

Detection of a Strange Particle
10 extraordinary papers Within days, Watson and Crick had built a identify the full set of codons was completed in forensics, and research into more-futuristic new model of DNA from metal parts. Wilkins by 1966, with Har Gobind Khorana contributing applications, such as DNA-based computing, immediately accepted that it was correct. It the sequences of bases in several codons from is well advanced. was agreed between the two groups that they his experiments with synthetic polynucleotides Paradoxically, Watson and Crick’s iconic would publish three papers simultaneously in (see go.nature.com/2hebk3k). structure has also made it possible to recog- Nature, with the King’s researchers comment- With Fred Sanger and colleagues’ publica- nize the shortcomings of the central dogma, ing on the fit of Watson and Crick’s structure tion16 of an efficient method for sequencing with the discovery of small RNAs that can reg- to the experimental data, and Franklin and DNA in 1977, the way was open for the com- ulate gene expression, and of environmental Gosling publishing Photograph 51 for the plete reading of the genetic information in factors that induce heritable epigenetic first time7,8. any species. The task was completed for the change. No doubt, the concept of the double The Cambridge pair acknowledged in their human genome by 2003, another milestone helix will continue to underpin discoveries in paper that they knew of “the general nature in the history of DNA. biology for decades to come. of the unpublished experimental results and Watson devoted most of the rest of his ideas” of the King’s workers, but it wasn’t until career to education and scientific administra- Georgina Ferry is a science writer based in The Double Helix, Watson’s explosive account tion as head of the Cold Spring Harbor Labo- Oxford, UK. -

Search for Supersymmetry Events with Two Same-Sign Leptons
Search for supersymmetry events with two same-sign leptons Dissertation der Fakult¨atf¨urPhysik der Ludwig-Maximilians-Universit¨atM¨unchen vorgelegt von Christian Kummer geboren in M¨unchen M¨unchen, im Januar 2010 1. Gutachter: Prof. Dr. Dorothee Schaile 2. Gutachter: Prof. Dr. Wolfgang D¨unnweber Tag der m¨undlichen Pr¨ufung: 09.03.2010 meiner Familie Abstract Supersymmetry is a hypothetic symmetry between bosons and fermions, which is broken by an unknown mechanism. So far, there is no experimental evidence for the existence of supersymmetric particles. Some Supersymmetry scenarios are predicted to be within reach of the ATLAS detector at the Large Hadron Col- lider. Final states with two isolated leptons (muons and electrons), that have same signs of charge, are suitable for the discovery of supersymmetric cascade decays. There are numerous supersymmetric processes that can yield final states with two same-sign or more leptons. Typically, these processes tend to have long cas- cade decay chains, producing high-energetic jets. Charged leptons are produced from decaying charginos and neutralinos in the cascades. If the R-parity is con- served and the lightest supersymmetric particle is a neutralino, supersymmetric processes lead to a large amount of missing energy in the detector. The most important Standard Model background for the same-sign dilepton channel is the semileptonic decay of top-antitop-pairs. One lepton originates from the leptonic decay of the W boson, the other lepton originates from one of the b quarks. Here, the neutrinos are responsible for the missing energy. The Standard Model background can be strongly reduced by applying cuts on the transverse momenta of jets, on the missing energy and on the lepton isolation. -

8.1 the Neutron-To-Proton Ratio
M. Pettini: Introduction to Cosmology | Lecture 8 PRIMORDIAL NUCLEOSYNTHESIS Our discussion at the end of the previous lecture concentrated on the rela- tivistic components of the Universe, photons and leptons. Baryons (which are non-relativistic) did not figure because they make a trifling contribu- tion to the energy density. However, from t ∼ 1 s a number of nuclear reactions involving baryons took place. The end result of these reactions was to lock up most of the free neutrons into 4He nuclei and to create trace amounts of D, 3He, 7Li and 7Be. This is Primordial Nucleosynthesis, also referred to as Big Bang Nucleosynthesis (BBN). 8.1 The Neutron-to-Proton Ratio Before neutrino decoupling at around 1 MeV, neutrons and protons are kept in mutual thermal equilibrium through charged-current weak interactions: + n + e ! p +ν ¯e − p + e ! n + νe (8.1) − n ! p + e +ν ¯e While equilibrium persists, the relative number densities of neutrons and protons are given by a Boltzmann factor based on their mass difference: n ∆m c2 1:5 = exp − = exp − (8.2) p eq kT T10 where 10 ∆m = (mn − mp) = 1:29 MeV = 1:5 × 10 K (8.3) 10 and T10 is the temperature in units of 10 K. As discussed in Lecture 7.1.2, this equilibrium will be maintained so long as the timescale for the weak interactions is short compared with the timescale of the cosmic expansion (which increases the mean distance between parti- cles). In 7.2.1 we saw that the ratio of the two rates varies approximately as: Γ T 3 ' : (8.4) H 1:6 × 1010 K 1 This steep dependence on temperature can be appreciated as follows.